Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489Print version ISSN 0038-2353
S. Afr. j. sci. vol.111 n.9-10 Pretoria Sep./Oct. 2015
https://doi.org/10.17159/SAJS.2015/20140212
RESEARCH ARTICLE
Nematode pests threatening soybean production in South Africa, with reference to Meloidogyne
Hendrika FourieI; Dirk de WaeleI, II; Alexander H. Mc DonaldI, †; Charlotte MienieI; Mariette MaraisIII; Annelie de BeerIV
IUnit for Environmental Sciences and Management, North-West University, Potchefstroom, South Africa
IIAfdeling Plantenbiotechniek, University of Leuven, Leuven, Belgium
IIIPlant Protection Research Institute, Agricultural Research Council, Pretoria, South Africa
IVGrain Crops Institute, Agricultural Research Council, Potchefstroom, South Africa
ABSTRACT
The area planted to soybean in South Africa has increased by 54% since the 2009 growing season, mainly as a result of the increasing demand for protein-rich food and fodder sources. Moreover, the introduction of advanced technology, namely the availability of genetically modified herbicide tolerant soybean cultivars also contributed towards increased soybean production. The omnipresence of plant-parasitic nematodes in local agricultural soils, however, poses a threat to the sustainable expansion and production of soybean and other rotation crops. Meloidogyne incognita and M. javanica are the predominant nematode pests in local soybean production areas and those where other grain-, legume- and/or vegetable crops are grown. The lack of registered nematicides for soybean locally, crop production systems that are conducive to nematode pest build-ups as well as the limited availability of genetic host plant resistance to root-knot nematode pests, complicate their management. Research aimed at various aspects related to soybean-nematode research, namely, audits of nematode assemblages associated with the crop, identification of genetic host plant resistance in soybean germplasm to M. incognita and M. javanica, the use of molecular markers that are linked to such genetic resistance traits as well as agronomic performance of pre-released cultivars that can be valuable to producers and the industry are accentuated in this review. Evaluation of synthetically-derived as well as biological-control agents are also discussed as complementary management tactics. It is important that lessons learned through extensive research on soybean-nematode interactions in South Africa be shared with researchers and industries in other countries as they might experience or expect similar problems and/or challenges.
Keywords: host plant resistance; legume; molecular markers; plant-parasitic nematodes; root-knot nematodes
Introduction
Soybean (Glycine max (L.) Merr) is a major source of protein and oil, both for local human and animal consumption.1,2 During the 2012/2013 growing season, sunflower ranked first in terms of its production (860 000 t)1, followed by soybean (710 000 t) and dry bean (60 200 t)3. These three are the main oilseed- and protein crops being produced in South Africa. Locally, the hectares planted to soybean have increased by 54% from 2008/2009 to the 2013/2014 growing seasons.1 Furthermore, the introduction of advanced technology in the form of genetically-modified, herbicide-tolerant, Roundup® Ready (RR) soybean material, was experienced in 2004 when such cultivars were released for commercial production in South Africa.4 These trends reflect the increasing and urgent need for oil and protein sources to feed a fast growing nation as well as its cattle industry.2
Although soybean was traditionally cultivated in the Free State, KwaZulu-Natal, Mpumalanga and Gauteng Provinces,1 its production was and still is extended to areas where predominantly maize and crops such as groundnut, sunflower, potato and others were traditionally grown. The initiative to expand and stimulate local soybean production resulted in exposure of the crop to new pests and diseases that have the potential to seriously reduce local soybean production.2 For example, the soybean leaf miner, Aproaerema modicella (Deventer) that was introduced into South Africa and reported as a pest of groundnut during the early 2000s also attacks soybean in certain areas of the country.5 A similar scenario was experienced in 2001 when the economically important soybean rust disease, caused by the newly-introduced fungus Phakopsora pachyrhizi Sydow, was first recorded as a major pathogen of soybean crops in traditional local production areas.6 Not only should soybean cultivars be adapted to local environmental conditions to optimise crop performance, it should also exhibit resistance to diseases and pests such as plant-parasitic nematodes, bacteria, fungi and insect pests.2
Although not always perceived as pests of soybean and other crops, plant-parasitic nematodes are economically one of the most important production constraints in crop production areas of sub-Saharan Africa.7 The latter include current and potential soybean production areas in South Africa.8-10 Towards the end of the 1980s, the estimated annual soybean yield losses resulting from plant-parasitic nematode parasitism amounted to approximately 9%.11 The 9%, however, referred to damage caused by various plant-parasitic nematode communities and did not distinguish between the contribution by particular nematode species. More recent assessments of the pest status of root-knot nematodes on soybean revealed yield losses that ranged from 25-70%.8,12,13 In addition, two of the national soybean cultivar trials that are annually conducted by the Agricultural Research Council's Grain Crops Institute (ARC-GCI) were terminated during 1999 as a result of high root-knot nematode infections causing total crop failure.14 Distinct root-galling (Figure 1a and b) represents below-ground symptoms and is caused by feeding of female root-knot nematodes (Figure 2). On the other hand, above-ground symptoms in fields where high population levels of these pests occur can include stunted plants with yellowing leaves (Figure 3).


The increased awareness and adverse impact of plant-parasitic nematodes on soybean crops and the expansion of the crop resulted in the initiation of several research projects. Subsequently, the significant body of knowledge regarding soybean-nematode interactions that was accumulated since the middle 1950s is discussed. As soybean production on the African continent increases, South African knowledge of this crop could be applied in the rest of Africa where soybean production is also increasing15 and where similar environmental conditions occur as those in South Africa. For these reasons, aspects are highlighted regarding the most important nematode pests of soybean, expected problems encountered with the introduction of soybean into production areas where it was not grown before as well as tactics that can be used to manage these pests.
Plant-parasitic nematodes associated with soybean in South Africa
To date, 18 plant-parasitic nematode genera and 48 species have been associated with soybean in South Africa (Table 1). In 1959, individuals of the endoparasitic root-knot nematode species M. arenaria were reported to parasitise soybean16, followed by listings of M. hapla, M. incognita and M. javanica being associated with the crop in 1968.17 During the early 1980's, the species list was extended when numerous plant-parasitic nematode species identified from rhisosphere soil and roots of soybean plants were added.18 Since then, more nematodes associated with soybean were reported from material deposited in the National Collection of Nematodes (Nematology Unit of the Agricultural Research Councils' Plant Protection Research Institution) and samples collected during surveys that formed part of the South African Plant-Parasitic Nematode Survey.9,19
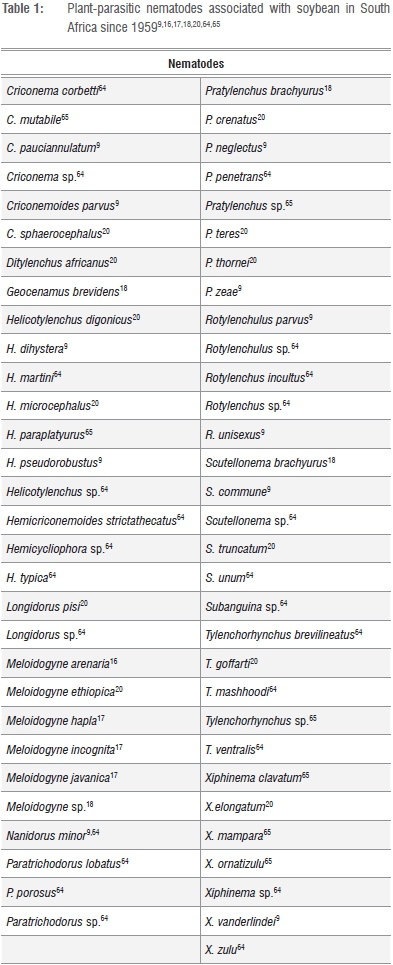
The first extensive nematode survey was conducted during 1995/1996 at 17 localities situated within the local soybean production areas.20 As a result, two nematode genera (Longidorus and Tylenchorhynchus) and 11 species were listed as new records for soybean in South Africa. The latter species were Criconemoides sphaerocephalus, Helicotylenchus digonicus, H. microcephalus, Longidorus pisi, Meloidogyne ethiopica Pratylenchus crenatus, P. teres, P. thornei, Scutellonema truncatum, Tylenchorhynchus goffarti, and Xiphinema elongatum. The predominant endoparasitic nematode pests identified from soybean roots during the survey were Meloidogyne spp. (M. ethiopica, M. hapla, M. incognita and M. javanica) and Pratylenchus spp. (P. brachyurus and P. zeae). Moreover, root-knot nematode second-stage juveniles (J2) were present in 91% of all root samples. It was also evident that the occurrence of the predominant endoparasitic nematodes was not restricted to sandy soil, but that they also occurred at localities containing soils with clay content as high as 35%. Although present in low population density levels, another economically important endoparasitic nematode species Ditylenchus africanus (peanut-pod nematode) was also identified from soybean roots. This nematode represents a definite production constraint for groundnut crops throughout local production areas.21 The soybean cyst nematode, Heterodera glycines, that poses a significant threat to soybean production in other parts of the world22,23 has, however, not been reported locally during the survey or to date (Marais M 2014, oral communication, June 6).
That root-knot nematodes are generally the predominant plant-parasitic nematodes associated with local soybean crops8,20 corresponds with reports that these pests are also considered as a serious constraint to production of the crop worldwide22,23. Diagnostic nematode analyses revealed exceptional high root-knot nematode population density levels of 11 401 eggs and J2/50 g roots from RR plants that grew in the Bothaville area (Free State Province) during the 2011 season.24 During April 2013, 161 213 Meloidogyne sp. eggs and J2/50 g roots were extracted from roots of a conventional soybean cultivar that was cultivated in the Edenville area (Free State Province).25 The latter areas include those to where soybean production has been expanded recently. Increased infection of soybean and rotation crops included in soybean-based cropping systems by single or mixed populations of M. incognita and M. javanica is thus imminent because of the damage potential of such pests. The latter species commonly occur in areas where soybean was traditionally cultivated in South Africa as well as in those areas where maize is grown8,10 and where soybean is now being introduced. This scenario particularly applies where soybean is included in conservation agriculture systems in which the use of herbicide tolerant cultivars is often preferred. To date, all genetically modified herbicide tolerant, RR soybean cultivars evaluated for their host suitability to M. incognita have been reported as susceptible.26,27 This scenario emphasises and complicates the challenge faced by producers and the industry to manage these pests in future. The emphasis on soybean-nematode research has been on the use and exploitation of genetic resistance as a viable and environmentally safe tactic to reduce population levels of particularly root-knot nematodes. Initiatives in this regard will be discussed below, followed by knowledge gained in terms of other management tactics that may add value to soybean producers and the industry.
Genetic resistance to root-knot nematodes
The use of root-knot nematode resistant soybean cultivars is one of the most economically justified strategies for controlling root-knot nematode pests.28 The rest of this review will thus focus on this strategy as the most popular, cost-effective and efficient strategy for sustainable production of soybean, while only a concise summary on other potential management strategies will be given.
The use of cultivars that exhibit resistance to root-knot nematodes generally results in substantial reductions in population levels of these pests.12,22,28 Despite the phenomenon that J2s penetrate roots of resistant cultivars to the same extent as that of susceptible cultivars, sub-optimal development of J2s within the roots of resistant host plants follow with subsequent retarded development of all J2 life-cycle stages.29-32 Significantly lower numbers of eggs are thus produced by mature root-knot nematode females that feed in roots of resistant cultivars opposed to those that parasitise roots of susceptible cultivars. Although a wide range of genotypes with varying levels of resistance to root-knot nematode species and races is available,23,23 such material is not necessarily adapted to local environmental conditions. They are also not necessarily resistant to local root-knot nematode species and races as will be illustrated below.
Studies on the host status of local soybean cultivars to root-knot nematodes were first reported in the 1990s when 19 commercially available cultivars were screened for their host suitability to M. javanica and M. incognita race 4, respectively.33 The cultivars differed with regard to their host suitability to the two respective root-knot nematode species, with relatively low to moderate levels of resistance being identified. During the end of the 1990s, further screening of local cultivars using various nematode life history parameters as criteria, namely egg-laying female indices, egg and J2 numbers/root system and reproduction factor (Rf) values, followed.34 The latter parameters varied substantially for the 38 soybean cultivars that were screened against M. incognita race 1, 2 and 4 as well as M. javanica. According to (Rf)values, none of the cultivars exhibited resistance to M. incognita race 2 (Table 2). However, several were considered to have some level of resistance to M. incognita races 1 and 4 as well as M. javanica (Table 2). Sources of resistance in local soybean cultivars against M. incognita races 2 and 4 and M. javanica (Table 2) were also reported during the mid 2000s.26,35 Of the 85 local and foreign soybean genotypes that were evaluated for host suitability to M. incognita race 2,26 LS5995 exhibited the highest level of resistance (Rf=0.01) followed by PI96354, PAN780, Egret, PAN660, LS688, Potties, PAN564, G93-9106, G93-9009, G93-9201 and LS666 (Table 2). Interestingly, Forrest that was recorded with partial resistance to USA populations of M. incognita,36proved to be susceptible to some local M. incognita race 2 populations.26,27

Although gall ratings and egg mass indices were commonly used as criteria for determining root-knot nematode resistance in soybean,28,37 egg production is generally regarded as a more reliable criterion.28,38 In some cases, soybean genotypes exhibited low gall ratings but high egg-laying female indices and high numbers of eggs/plant. This unexpected crop reaction to root-knot nematode infection was also reported for exotic soybean cultivars.36 It implies that using gall ratings alone can lead to inaccurate interpretation of data regarding cultivar resistance to root-knot nematodes. This phenomenon is further illustrated as plant resistance could be affected through one or several different mechanisms of resistance.28,37 Several criteria describing the possible resistance mechanism involved should thus be applied during the identification of resistance in crop cultivars. Results obtained during screenings, however, ultimately resulted in valuable knowledge being available for use in the planning of crop rotation systems as well as the exploitation of sources of resistance for breeding purposes. Undoubtedly, the continuous screening of cultivars that enter the market is crucial because producers should be updated annually on poor-host cultivars that could be used in their rotation systems. In this way, root-knot nematode populations can be reduced on a continuous basis to allow for the sustainable production of crops.
Molecular markers linked to root-knot nematode resistance
Molecular methods, i.e. marker-assisted selection (MAS) during breeding, have been applied widely to improve the success rate and levels of root-knot nematode resistance selection28 and to accelerate the development of soybean cultivars that exhibit this trait.39-44 Genetic markers associated with resistance to M. incognita41-43, M. javanica39,44and M. arenaria40have been identified using amplified fragment length polymorphism (AFLP), restriction fragment length polymorphism (RFLP), sequence characterised amplified regions (SCAR) and/or micro-satellite or simple sequence repeat (SSR) markers. The use of such markers linked to root-knot nematode resistance traits in soybean cultivars and subsequent application of MAS is a quick and effective way to expedite nematode resistance breeding processes28,39-44, which has also been exploited in local resistance breeding programmes45,46. The latter include identification and verification of M. javanica45and M. incognita resistance46, being additional milestones for soybean-nematode research in South Africa.
Meloidogyne javanica
AFLP markers linked to M. javanica resistance in the local soybean cultivar Gazelle and subsequent conversion thereof to SCARS45, was the first successful attempt for such research on soybean in South Africa. A close linkage of RFLP marker B212 was reported for the resistance trait, accounting for 62% of the variation in M. javanica gall index measurements. Marker data obtained in this regard corresponded with those for a marker located in the same region on LG-F for the exotic M. javanica-resistant soybean line PI230977.39 However, the other marker, A725-2 situated on LG-D1, that accounts for only 13% of gall index variation in the latter exotic line, was not polymorphic for the two parents used in the local mapping population and thus not detected in Gazelle.
The AFLP fragments identified in Gazelle were then used to develop a marker system that is easily and economically applicable in MAS in local breeding programmes.45 Marker E-AAC/M-CAT1 (LG-F) that linked in the repulsion phase accounted for the greatest variation in gall indices (42%), while marker E-ACC/M-CTC2 (LG-F) that linked in the coupling phase explained 25% of the variation for the same nematode parameter. Both markers associated with the gall index parameter were linked to LG-F. The quantitative trait locus (QTL) for gall-index resistance mapped between markers B212 and E-AAC/M-CAT1 (SOJA7), which according to MAPMAKER-EXP analysis are only 2.4 cM apart. E-ACC/M-CTC2 (SOJA6), mapped near B212, with the QTL being recorded as 3.8 cM from marker B212. The latter indicates that the combined use of these two markers in MAS could be very effective. Subsequently the two AFLP markers mapping closely to and bracketing the M. javanica resistance trait in Gazelle were successfully converted to SCARs (SOJA6 and SOJA7, respectively) and employed for MAS in a breeding population. SOJA6 distinguished between homozygotic and heterozygotic progeny and SOJA7 against homozygous resistant plants only. Successful conversion of AFLP markers to either RFLP or polymerase chain reaction (PCR) markers has been reported by only a few authors.47-50 Additional QTLs, if present, would likely be of minor importance in terms of a contribution to explaining variation in gall index for M. javanica resistance in local cultivar Gazelle.
Meloidogyne incognita
For identification of molecular markers associated with M. incognita resistance, the soybean cultivar LS5995 was used as the resistant parent in crosses to obtain a segregating F2 mapping population.46 A lack of polymorphism for SSR markers between the parents, however, complicated the identification and location of QTLs associated with the M. incognita resistance in LS5995. As a result of the lack of polymorphism, accurate mapping of a major QTL associated with resistance to M. incognita in the resistant USA soybean line PI9635441 could not be achieved for the local resistant LS5995. The F2 population was screened with a number of SSR evenly distributed throughout the soybean genome, with Satt201, Satt358, Satt487 and Satt590 being the SSR markers identified as those linked to the resistance trait.46 QTL data obtained for LS5995 differed from those published by other authors41,42 because no SSR markers could be identified in association with resistance to M. incognita in LS5995 on LG-G. In contrast, a minor QTL (Satt012) on LG-G that explained 18% of the variation in M. incognita gall indices41 as well as three SNPs in Satt199 source-sequences were linked near a major QTL in the exotic line PI96354.42 In the local F2 mapping population, two QTLs were identified using variation in gall indices and egg and J2 numbers/root system. One of these was located on LG-O (close to the one identified for the exotic line PI96354),42 while the other major QTL was located on LG-M. The QTL located near Satt358 explained 56% of the variation in gall indices on LG-O for PI96354, while in local cultivar LS5995, it only explained 32% of the phenotypic variation for this parameter. On the other hand, the major QTL on LG-M identified in this study explained 80% of the variation in M. incognita eggs and J2 numbers/root system. The latter QTL was not identified in the exotic line PI96354,42 suggesting that different resistance mechanisms are involved in the two genotypes. The frequency distribution of the F2 progeny for both M. incognita gall indices and eggs and J2 numbers/ root system suggested that resistance in LS5995 was quantitatively inherited and is thus controlled by a number of genes and not by partially dominant inheritance of one major gene.51 Validation of molecular markers associated with resistance to M. incognita present in local and foreign cultivars and an F6 progeny of a LS5995 x Prima2000 cross52 further emphasises and confirms the value of MAS in soybean breeding programmes. Important to note, however, is that the presence of only one of the several markers identified in a particular root-knot nematode-resistant cultivar does not necessarily guarantee the introgression of such resistance. For example, the M. incognita-resistant soybean cultivar Forrest contains three markers (Satt201, Satt487 and Satt358)46 but proved to be susceptible when screened to a local population of this species in several glasshouse experiments46,34. This scenario also emphasises the use of MAS in combination with traditional screening procedures to ensure that resistance is successfully introgressed into a genotype.
Ultimately, interventions to pyramid both minor and major genes linked to M. incognita and M. javanica resistance should enhance the level of these traits in local soybean cultivars. This way, superior and polyspecific levels of root-knot nematode resistance can be selected for in germplasm.
Resistance mechanism(s)
The resistance mechanism(s) exhibited by the M. incognita-resistant cultivar LS5995 was determined by means of penetration, development, reproduction and histopathology studies.29 These studies showed that J2s initially penetrated roots of the resistant LS5995 and susceptible Prima2000 in equal numbers. However, the J2 penetration rate was significantly lower in roots of LS5995 10 days after inoculation, which corresponds with results for exotic M. incognita-resistant genotypes reported by other authors.30-32 Furthermore, numbers of J2 developmental stages were 4.6-fold higher in the roots of the susceptible Prima2000 compared to that in LS5995. Ultimately, the number of eggs/egg mass and numbers of eggs/root system, which are important indicators of antibiosis resistance, were also significantly lower in LS5995. These studies therefore indicated that the major mechanism of resistance in the resistant cultivar represented typical post-infectional antibiosis.
Histopathology investigations on the other hand illustrated that M. incognita J2s penetrated roots of both the resistant and susceptible cultivars and migrated intercellularly to the parenchyma cells in the vascular cylinder 2 days after inoculation.29 Pronounced cellular changes were also observed in the roots of both cultivars between 10 and 30 days after inoculation and generally represented those reported for other exotic resistant and susceptible cultivars.53,54 The presence of sub-optimal giant cells, some with distinctly thicker cell walls that was recorded for the resistant LS5995 had not been reported for other resistant soybean cultivars or resistant cultivars of other crops before.53,54
Whether the presence of such atypical giant cells with thicker giant cell walls in roots of LS5995 can be ascribed to differences in genetic markers associated with M. incognita resistance in LS5995 compared to those reported in exotic cultivars42 is unknown and warrants further investigation. Giant cells in the roots of the resistant LS5995 were also smaller and fewer (Figure 4) compared to those in roots of its susceptible counterpart (Figure 5). The association of M. incognita individuals that showed retarded development with sub-optimal giant cell formation (including necrosis around the giant cells; Figure 6) and ultimately reduced reproduction and fecundity in LS5995, further illustrated the presence of multiple defence strategies at genetic level to withstand parasitism by M. incognita. These findings also complemented the quantitative nature of the resistance identified in LS5995. Finally, studies at cellular level substantiated and gave insight into host plant defence mechanisms employed in LS5995. This can contribute to enhancing the development of strategies to better understand and engineer resistance against this species.



Verification of resistance
Damage-threshold levels
The damage-threshold level that was determined for M. incognita in the resistant cultivar LS5995 in semi-field studies was 10 times higher compared to that of its susceptible counterpart.12,55 This study illustrated that a relatively small economic loss will be sustained when a resistant cultivar is planted compared to a susceptible one. However, to extrapolate and apply such information to other areas is complicated because numerous factors have an effect on nematode threshold levels, such as environmental conditions, the cultivar planted, the nematode species/race present as well as other geographic and edaphic factors.56 Therefore, it is suggested that damage-threshold values for root-knot nematodes in agricultural crops, soybean in particular, should be considered circumspectively and at most, be used as guidelines for implementation of control action. This implies that successful management of root-knot nematodes in soybean cannot be done by using a single strategy such as host plant resistance. Additional nematode control strategies such as chemical control (if available), cultural control and others should for example be used on an integrated basis to ensure effective long term suppression of nematode populations.
Glasshouse and semi-field studies
Resistance against M. incognita was verified by determining the effect of increasing initial population density levels (Pi) on population dynamics and yield of the resistant LS5995 as well as a susceptible counterpart in semi-field studies.12 Strong, non-linear relationships existed between Pi for all the nematode variables used, namely, number of egg masses, egg-laying females indices, Rf-values and percentage yield loss. Non-linear models for Pi against percentage yield loss indicated that yield loss in LS5995 was at least six times lower than that of the susceptible cultivar, which demonstrated the monetary benefit provided by the resistant cultivar. Similar results and benefits of differential yield loss in exotic resistant soybean cultivars grown in M. incognita-infested soil have been reported.57
Distinct differences in the response of M. incognita individuals in terms of their life history parameters, furthermore confirmed the resistance trait in LS5995.29 The importance of considering as many as possible nematode parameters when investigating aspects such as host plant resistance was highlighted as a result of this research. It is also suggested that the use of host plant resistance in only one crop (soybean in this case) in local rotation cycles with other susceptible crops such as maize8,10, sunflower58, potato59 or dry bean9,11 may not be sufficient for keeping M. incognita population levels below damage-threshold levels in the medium to long term. Also, the use of host-plant resistance as the only management strategy may not provide sufficient protection against nematode pests. Therefore, careful consideration of as many factors as possible during the planning and development of production systems where nematode-susceptible crops will be cultivated in root-knot nematode infested soils needs to be considered.
Field studies
Host and yield responses of M. incognita-resistant genotypes identified in initial screenings, including LS5995, together with two susceptible soybean cultivars were furthermore verified under natural environmental conditions.55 Root-knot nematode numbers in both soil and root samples were significantly higher for all genotypes inoculated with M. incognita eggs and J2 compared to the uninoculated control plants. Furthermore, the number of eggs and J2 in the roots of nematode-infected plants was significantly higher in the susceptible cultivars compared to the resistant genotypes, except for the resistant cultivar Potties in one of the trial sites. In contrast with the high reproduction of root-knot nematodes in roots of the susceptible Prima/Prima2000, LS5995 in particular, consistently maintained significantly lower M. incognita population levels in all field experiments. Also, in the majority of the experiments, yield of the resistant genotypes did not differ significantly between the uninoculated and the nematode-infected plants. Yield response was, however, generally dependent on environmental effects, as was also indicated by authors,56,57 and thus limited further qualification of resistant or susceptible soybean genotypes as tolerant, intolerant and/or hypersensitive.
Eight F8 lines with superior levels of resistance to M. incognita52, resulting from breeding efforts to identify molecular markers in the local resistant cultivar LS5995 were additionally evaluated for their agronomic performance during two seasons at eight different localities60. Yield data of two of these lines were similar to that of Egret, which is the only cultivar with resistance to M. incognita that is currently available commercially. These genotypes do not contain the RR gene and no such root-knot nematode-resistant soybean cultivars are registered at this stage in South Africa, therefore, exploitation of such material and conversion thereof to RR material will add value to the local soybean industry as an investment to complement sustainable production of the crop.
Other management strategies
Chemical and biological control
Although several nematicides are registered for use on soybean worldwide22,23, but not in South Africa61, application of such products is seldom economically justifiable22,23. Locally, evaluation of nematicides on soybean that was cultivated in M. javanica-infested soil included ethylene-dibromide (EDB®), aldicarb and chlorpirifos as well as two biological products. The latter contained Paecelomyces lilacinus and Bacillus spp., respectively.62 Plots where the soil was fumigated with EDB® consistently resulted in the lowest M. javanica population levels and highest yields, followed by those treated with aldicarb and terbufos. The bionematicide treatments did, however, not always differ significantly from the untreated control or other treatments in terms of their efficacy.
Nematicide efficacy was also conducted under field conditions where high infestation levels of M. incognita were present.63 Fourteen nematicide treatments were included and represented various dosages of aldicarb, abamectin, cadusafos, oxagran, oxamyl and terbufos. Significant differences in efficacy of nematicides existed with regard to the number of egg and J2 counts/50 g roots at all localities. Only oxamyl SL, terbufos GR and abamectin/seed treatments resulted in a significant reduction of M. incognita numbers in 50g roots and generally showed a higher income/ha compared to the untreated control. Although data from these trials indicated that synthetically-derived nematicides may provide relief to producers where root-knot nematodes attain high pest status, cost-efficacy analyses did not allow registration of these products for use in local soybean production systems at the time. However, the evaluation of newly-developed products with nematicidal properties should proceed. The use of sustainable strategies where such products can contribute to reduce plant-parasitic nematodes in an integrated management approach can be advantageous to producers.
Conclusion
The magnitude of plant-parasitic nematode problems, focussing on root-knot nematodes, in soybean has been illustrated in this article. Sustainable production of soybean is likely to be jeopardised as a result of the build-up of various root-knot nematode species, in particular, in soybean-based cropping systems. This necessitates research aimed at quantifying the impact of these and other nematode pests in producing areas where cultivation of the crop is planned. In addition, it is crucial that funding be secured on a continuous basis to develop improved management systems for these pests in soybean-based cropping systems. Current approaches towards environmentally-friendly strategies to combat plant-parasitic nematodes increase the pressure on researchers and decision-makers in the soybean industry to coordinate research initiatives and seek sustainable solutions. Future research should particularly address the identification of alternative sources of resistance to economically important plant-parasitic nematodes, such as Meloidogyne spp. In addition, the development of integrated strategies to combat nematode pests of soybean should be addressed.
Acknowledgements
The research was partially financed by the Protein Research Foundation, while the North-West University and Agricultural Research Council-Grain Crops Institute supplied the infrastructure. The following research assistants provided valuable support and inputs during the execution of research activities related to this manuscript: Samuel Kwena, Rita Jantjies, Lizette Bronkhorst, Nicolene de Klerk and Heila Vermeulen from the Agricultural Research Council-Grain Crops Institute as well as Edith du Randt, Emily Phetoe and Helena Strydom from the North-West University. The financial assistance of the National Research Foundation towards this research is hereby also acknowledged. Opinions expressed and conclusions arrived at, are those of the author and are not to be attributed to the NRF.
Authors' contributions
H.F. initiated the writing of this review article and compiled the initial manuscript. As the promotor and co-promoter of H.F.'s MSc and PhD studies, respectively, which represents a major part of nematology research done on soybean locally, D.d.W. and A.H.M. edited and provided valuable inputs to the manuscript. C.M. is the molecular specialist that was involved in the execution of the molecular identification of genetic markers associated with the resistance in soybean cultivars to two Meloidogyne species. M.M is a nematode taxonomist who identified plant-parasitic nematodes associated with soybean to species level and supplied the information from the South African Plant-Parasitic Nematode Survey database about plant-parasitic nematodes reported from soybean in South Africa. A.D. has been involved in executing field trials to assess yield and other data on the agronomic performance of S8 progeny of soybean lines in which Meloidogyne incognita and M. javanica resistance has been introgressed by H.F. and a soybean breeder (Pieter Herbst from the former LinkSeed). M.M. and A.D. also contributed towards inclusion of data and information and the final editing of the manuscript.
References
1. Protein Research Foundation (PRF). Statistics and estimates [homepage on the Internet]. [ Links ] No date [cited 2014 Mar 27]. Available from: http://www.proteinresearch.net/index.php?dirname=html_docs_030statistics_and_estimates
2. Liebenberg A. Soybean production manual: Your guide to successful soybean production. Potchefstroom: Agricultural Research Council; 2012. [ Links ]
3. South African Grain Information Service (SAGIS). CEC crop estimates [homepage on the Internet]. [ Links ] No date [cited 2014 Mar 27]. Available from: http://www.sagis.org.za//Flatpages/Oesskatting.htm;2013.
4. James C. International service for the acquisition of Agri-biotech applications (ISAAA). Report on global status of biotech/GM crops [homepage on the internet]. [ Links ] c2009 [cited 2013 Nov 23]. Available from: http://www.isaaa.org/.
5. Du Plessis H. First report of groundnut leafminer Aproaerema modicella (Deventer) (Lepidoptera: Gelechiidae) on groundnut, soybean and lucerne in South Africa. S Afr J Plant Soil. 2001;20(1):48. http://dx.doi.org/10.1080/02571862.2003.10634906 [ Links ]
6. Jarvey JA. A review on soybean rust from a South African perspective. SA J Soil Sci. 2009;105:103-108. [ Links ]
7. Coyne DL, Fourie HH, Moens M. Current and future management strategies in resource-poor farming. In: Perry R, Moens M, Starr JL, editors. Root-knot nematodes. Wallingford: CABI; 2009. p. 444-475. http://dx.doi.org/10.1079/9781845934927.0444 [ Links ]
8. Riekert HF, Henshaw GE. Effect of soybean, cowpea and groundnut rotations on root-knot nematode build-up and infestation in dryland maize. Afr Crop Sci J. 1998;6:377-383. http://dx.doi.org/10.4314/acsj.v6i4.27789 [ Links ]
9. Kleynhans KPN, Van den Berg E, Swart A, Marais M, Buckley NH. Plant nematodes in South Africa. Pretoria: Business Print; 1996. [ Links ]
10. Riekert HF. Economic feasibility of nematode control in dryland maize in South Africa. Afr Crop Sci J. 1996;4:477-481. [ Links ]
11. Keetch DP. A perspective of plant nematology in South Africa. S Afr J Sci.1989;85:506-508. [ Links ]
12. Fourie H, Mc Donald AH, De Waele D. Relationships between initial population densities of Meloidogyne incognita race 2 and nematode population development in terms of variable soybean resistance. J Nematol. 2010;42:55-61. [ Links ]
13. Fourie H, Mc Donald AH. Control of root-knot nematodes on soybean. Presented at the Fifteenth Symposium of the Nematological Society of Southern Africa. Afr Plant Prot. 2002;8:79-80. [ Links ]
14. Smit MA, De Beer GP. Report of the national soybean cultivar trials 1998/99. Potchefstroom: Agricultural Research Council; 1998. [ Links ]
15. Food Agricultural Organization (FAO). Food and Agriculture Organization of the United Nations [homepage on the Internet]. [ Links ] No date [cited 2014 Mar 27]. Available from: www.faostat.fao.org/
16. Van der Linde WJ, Clemitson JG, Crous ME. Host-parasite relationships of South African root-knot eelworm (Meloidogyne spp.). Dep Agric Techn Serv Repub S Afr Ent Ser. 1959;44:3-16. [ Links ]
17. Coetzee v. The distribution of the family Heteroderidae (Filipjev, 1934) in South Africa and some host records of Meloidogyne species. S Afr J Agr Sci. 1968;11:775-788. [ Links ]
18. Keetch DP, Buckley NH. A check-list of the plant-parasitic nematodes of southern Africa: Technical Communication No. 195. Pretoria: Department of Agriculture, Government Printer; 1984. [ Links ]
19. Marais M. South African plant-parasitic nematode survey (SAPPNS). Plant Prot News. 2006;67:6. [ Links ]
20. Fourie H, Mc Donald AH, Loots GC. Plant-parasitic nematodes in field crops in South Africa 6: Soybean. Nematology. 2001;3:447-454. http://dx.doi.org/10.1163/156854101753250773 [ Links ]
21. De Waele D, Jones BL, Bolton C, Van Den Berg E. Ditylenchus destructor in hulls and seeds of peanut. J Nematol. 1989;21:10-15. [ Links ]
22. Bridge J, Starr JL. Plant nematodes of agricultural importance. Boston, MA: Academic Press; 2007. http://dx.doi.org/10.1201/b15142 [ Links ]
23. Sikora RA, Greco N, Silva JFV. Nematode parasites of food legumes. In: Luc M, Sikora RA, Bridge J, editors. Plant parasitic nematodes in subtropical and tropical agriculture. Wallingford: CABI; 2005. p. 259-318. http://dx.doi.org/10.1079/9780851997278.0319 [ Links ]
24. Fourie H, Bekker S, Mc Donald AH, Engelbrecht E. Prospective extended benefits of plant-nematode diagnostic and advisory services should closer cooperation between laboratories be attainable. Presented at the Twentieth NSSA Symposium. S Afr J Plant Soil. 2011;28(4):265. [ Links ]
25. Fourie H. Research Report No 3: Nematode survey (2012/2013) in local soybean production areas. Potchefstroom: Nematology Unit, Plant Protection, North-West University; 2014. [ Links ]
26. Fourie H, Mc Donald AH, De Waele D. Host suitability of South African and foreign soybean cultivars to Meloidogyne incognita race 2. S Afr J Plant Soil. 2006;23:132-137. http://dx.doi.org/10.1080/02571862.2006.10634743 [ Links ]
27. Venter C. Exploitation and characterisation of resistance to the root-knot nematode Meloidogyne incognita in soybean [MSc dissertation]. Potchefstroom: North-West University; 2014. [ Links ]
28. Starr JL, Mercer CF. Development of resistant varieties. In: Perry R, Moens M, Starr JL, editors. Root-knot nematodes. Wallingford: CABI; 2009. p. 326337. http://dx.doi.org/10.1079/9781845934927.0326 [ Links ]
29. Fourie H, Mc Donald AH, De Waele D, Jordaan A. Comparative cellular responses in resistant and susceptible soybean cultivars infected with Meloidogyne incognita. Nematology. 2013;15:695-708. http://dx.doi.org/10.1163/15685411-00002712 [ Links ]
30. Moura RM, Davis EL, Luzzi BM, Boerma HR, Hussey RS. Post-infectional development of Meloidogyne incognita on susceptible and resistant soybean genotypes. Nematropica. 1993;23:7-13. [ Links ]
31. Hadisoeganda WW, Sasser JN. Resistance of tomato, bean, southern pea, and garden pea cultivars to root-knot nematodes based on host suitability. Plant Dis. 1982;66:145-150. http://dx.doi.org/10.1094/PD-66-145 [ Links ]
32. Kaplan DT, Davis EL. Mechanisms of plant incompatibility with nematodes. In: Veech JA, Dickson W, editor. Vistas on nematology. Hyattsville, MD: Society of Nematologists Inc.; 1987. p. 267-276. [ Links ]
33. Van den Berg E, Mc Donald AH. Screening of soybean cultivars for resistance against M. javanica and M. incognita race 4. Presented at the Tenth Symposium of the Nematological Society of Southern Africa; 1991 Apr 7-10; Wilderness, South Africa. Available from: http://www.sanematodes.com/Documents/Abstracts1991.pdf.34. [ Links ]
34. Fourie H, Mc Donald AH, Loots GC. Host suitability of South African commercial soybean cultivars to two root-knot nematode species. Afr Plant Prot. 1999;2:119-124. [ Links ]
35. Van Biljon ER. Crop rotation as part of an integrated pest management programme for the control of plant parasitic nematodes. Presented at: The Fourteenth Soilborne Plant Diseases Interest Group Symposium; 2004 Sept 15-16; Stellenbosch, South Africa. Available from: http://www.saspp.co.za. [ Links ]
36. Luzzi BM, Boerma HR, Hussey RS. Resistance to three species of root-knot nematode in soybean. Crop Sci. 1987;27:258-262. http://dx.doi.org/10.2135/cropsci1987.0011183X002700020027x [ Links ]
37. Cook R, Starr JL. Resistant cultivars. In: Perry R, Moens M. Plant nematology. Wallingford: CABI; 2006. p. 370-391. http://dx.doi.org/10.1079/9781845930561.0370 [ Links ]
38. Windham GL, Williams WP. Reproduction of Meloidogyne javanica on corn hybrids and inbreds. Ann Appl Nematol. 1988;2:25-28. [ Links ]
39. Tamulonis JP Luzzi BM, Hussey RS, Parrott WA, Boerma HR. DNA markers associated with resistance to Javanese root-knot nematode in soybean. Crop Sci. 1997;37:783-788. http://dx.doi.org/10.2135/cropsci1997.0011183X003700060039x [ Links ]
40. Tamulonis JP, Luzzi BM, Hussey RS, Parrott WA, Boerma HR. DNA marker analysis of loci conferring resistance to peanut root-knot nematode in soybean. Theor Appl Genet. 1997;95:664-670. http://dx.doi.org/10.1007/s001220050610 [ Links ]
41. Tamulonis JP Luzzi BM, Hussey RS, Parrott WA, Boerma HR. RFLP mapping of resistance to southern root-knot nematode in soybean. Crop Sci. 1997;37:1903-1909. http://dx.doi.org/10.2135/cropsci1997.0011183X003700060039x [ Links ]
42. Li Z, Jakkula L, Hussey RS, Tamulonis JP. SSR mapping and confirmationof QTL from PI96354 conditioning soybean resistance to southern root-knot nematode. Theor Appl Genet. 2001;103:1167-1173. http://dx.doi.org/10.1007/s001220100672 [ Links ]
43. Ha BK, Hussey RS, Boerma HR. Development of SNP assays for marker-assisted selection of two southern root-knot nematode resistance QTL in soybean. Crop Sci. 2007;47:73-82. http://dx.doi.org/10.2135/cropsci2006.10.0660tpg [ Links ]
44. Fuganti R, Beneventi MA, Silva JFV Arias CAA, Silvana RR, Binneck ME, et al. Identification of microsatellite markers for the selection of soybean genotypes resistant to Meloidogyne javanica. Nematol Bras. 2004;28:125-130. [ Links ]
45. Mienie CMS, Fourie H, Smit MA, Van Staden J, Botha FC. Identification of AFLP markers in soybean linked to resistance to Meloidogyne javanica and conversion to sequence characterized amplified regions (SCARS). Plant Growth Regul. 2002;37:157-166. http://dx.doi.org/10.1023/A:1020585023976 [ Links ]
46. Fourie H, Mienie CMS, Mc Donald AH, De Waele D. Identification of genetic markers associated with Meloidogyne incognita race 2 resistance in soybean (Glycine max L. Merr). Nematology. 2008;10:651-661. http://dx.doi.org/10.1163/156854108785787235 [ Links ]
47. Cho YG, Blair WM, Panaud O, McCouch SR. Cloning and mapping of variety-specific rice genomic DNA sequences: Amplified fragment length polymorphisms (AFLP) from silver-stained polyacrylamide gels. Genome. 1996;39:373-378. http://dx.doi.org/10.1139/g96-048 [ Links ]
48. Meksem K, Leister D, Peleman J, Zabeau M, Salamini F, Gebhardt C. A high-resolution map of the vicinity of the R1 locus on chromosome V of potato based on RFLP and AFLP markers. Mol Gen Genet. 1995;249:74-81. http://dx.doi.org/10.1007/BF00290238 [ Links ]
49. Qu LJ, Foote TN, Roberts MA, Aragon-Alcaide L, Snape JW, Moore G. A simple PCR-based method for scoring the ph1b deletion in wheat. Theor Appl Genet. 1998;96:371-375. http://dx.doi.org/10.1007/s001220050751 [ Links ]
50. Shan X, Blake TK, Talbert LE. Conversion of AFLP markers to sequence-specific PCR markers in barley and wheat. Theor Appl Genet. 1999;98:1072-1078. http://dx.doi.org/10.1007/s001220051169 [ Links ]
51. Fehr WR. Principles of cultivar development: Vol. 1: Theory and technique. New York: Macmillan; 1987. [ Links ]
52. Fourie H, Mc Donald AH. Report on project O14/01: Introduction of root-knot nematode resistance into local, popular soybean genotypes using marker-assisted selection (MAS) and enhanced generation advancement. Potchefstroom: Agricultural Research Council - Grain Crops Institute; 2010. p. 8-9. [ Links ]
53. Barker KR, Hussey RS. Histopathology of nodular tissues of legumes infected with certain nematodes. Phytopathology. 1976;66:851-855. http://dx.doi.org/10.1094/Phyto-66-851 [ Links ]
54. Pedrosa EMR, Hussey RS, Boerma HR. Penetration and post-infectional development and reproduction of Meloidogyne arenaria races 1 and 2 on susceptible and resistant soybean genotypes. J Nematol. 1996;28:343-351. [ Links ]
55. Fourie H, Mc Donald AH, De Waele D. In vivo host and yield responses of Meloidogyne incognita-resistant and susceptible soybean genotypes. Int J Pest Manag. 2013;59(2):111-121. http://dx.doi.org/10.1080/09670874.2013.772261 [ Links ]
56. Barker KR, Olthof THA. Relationships between nematode population densities and crop response. Annu Rev Phytopathol. 1976;14:327-353. http://dx.doi.org/10.1146/annurev.py.14.090176.001551 [ Links ]
57. Herman M, Hussey RS, Boerma HR. Response of resistant soybean plant introductions to Meloidogyne incognita in field microplots. J Nematol. 1990;22:237-241. [ Links ]
58. Bolton C, De Waele D, Loots GC. Plant-parasitic nematodes on field crops in South Africa 3: Sunflower. Rev Nématol. 1989;12:69-76. [ Links ]
59. Onkendi EM, Moleleki LN. Distribution and genetic diversity of root-knot nematodes (Meloidogyne spp.) in potatoes from South Africa. Plant Pathol. 2013;62(5):1184-1192. http://dx.doi.org/10.1111/ppa.12035 [ Links ]
60. De Beer A, Fourie H, Venter C, De Klerk N. Evaluation of root-knot nematode (Meloidogyne incognita) resistant soybean genotypes for their host status and yield potential. Presented at: The Combined Congress; 2014 Jan 20-23; Grahamstown, South Africa. p. 39. [ Links ]
61. Van Zyl K. A guide to crop pest management in South Africa. A compendium of acaracides, insecticides, nematicides, molluscicides, avicides and rodenticides. A CropLife South African Compendium. 1st ed. Pinetown: VR Print; 2013. [ Links ]
62. Fourie H, Mc Donald AH. Report for Project M151/60: Chemical control options for plant-parasitic nematodes associated with soybean in South Africa. Potchefstroom: Agricultural Research Council - Grain Crops Institute; 2001. [ Links ]
63. Fourie H, Mc Donald AH. Chemical control options for plant-parasitic nematodes associated with soybean in South Africa: Report for Project M151/60. Potchefstroom: Agricultural Research Council - Grain Crops Institute; 2007. [ Links ]
64. Marais M. Identification job sheet N3092: Dataset from South African Plant-Parasitic Nematode Survey database. Pretoria: Nematology Unit, Biosystematics Division, Plant Protection Research Institute, Agricultural Research Council; 2012. [ Links ]
65. Marais M, Swart A. Plant nematodes in South Africa 8: Bizana, Lusikisiki and Port St Johns area, Eastern Cape Province. Afr Plant Prot. 2007;13:16-27. [ Links ]
66. De Beer, G Pretorius, AJ Fourie H. Soybean cultivar recommendations for 2001/2002. Potchefstroom: Agricultural Research Council - Grain Crops Institute; 2002. [ Links ]
 Correspondence:
Correspondence:
Hendrika Fourie
Unit for Environmental Sciences
and Management, North-West
University, Private Bag X6001
Potchefstroom 2520
South Africa
Email: driekie.fourie@nwu.ac.za
Received: 19 June 2014
Revised: 21 Oct. 2014
Accepted: 19 Jan. 2015
† Deceased















