Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Orthopaedic Journal
On-line version ISSN 2309-8309Print version ISSN 1681-150X
SA orthop. j. vol.19 n.3 Centurion Aug./Sep. 2020
https://doi.org/10.17159/2309-8309/2020/v19n3a8
CURRENT CONCEPTS REVIEW
Maré PHI; Thompson DMII
IMBChB, FCOrth(SA); Head Clinical Unit: Paediatric Orthopaedics, Grey's Hospital, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Pietermaritzburg, South Africa
IIMBChB, FCS(Glasgow); Specialist orthopaedic surgeon, Grey's Hospital, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Pietermaritzburg, South Africa
ABSTRACT
Infantile Blount's disease results in multi-planar proximal tibial deformity consisting of varus, procurvatum, internal rotation and shortening. The deformity is attributed to disordered growth of the posteromedial proximal tibial physis.
The aetiology is multifactorial. It is associated with childhood obesity and African ethnicity. The ability to differentiate between infantile Blount's disease and physiological bowing depends on the findings of focused clinical examination, X-ray appearance, tibial metaphyseal-diaphyseal angle and tibial epiphyseal-metaphyseal angle.
The gold standard of treatment is proximal tibial metaphyseal corrective osteotomy before the age of 4 years. The limb should be realigned to physiological valgus.
The recurrence rate after realignment osteotomy is high. Recurrence is associated with age at osteotomy, obesity, higher Langenskiöld stage and medial physeal slope >60°.
The surgical management of severe, recurrent or neglected infantile Blount's disease is challenging. Comprehensive clinical examination and multi-planar deformity analysis with standing long leg X-rays are essential to identify all aspects of the deformity. Distal femur coronal malalignment and significant rotational deformity should be excluded. Knee instability due to intra-articular deformity should be corrected by elevation of the medial tibial plateau.
Lateral epiphysiodesis should be done at the same time as medial plateau elevation and when medial growth arrest is certain to prevent recurrence.
Level of evidence: Level 5
Keywords: Blount's disease, tibia vara, genu varum, recurrence, obesity
Introduction
Blount's disease is an abnormality of growth of the metaphysis, epiphyseal cartilage and osseous centre of the epiphysis.1 The consequence is abrupt angulation of the proximal tibia into varus,2 in association with procurvatum and internal tibial torsion. Associated deformities may occur in the femur and hindfoot. The disorder was first described by Erlacher in 1922.1 Blount was the first to provide a detailed description of the clinical features, X-ray findings and treatment of 28 cases of tibia vara. Subsequently the disorder is most commonly known as Blount's disease or alternatively as tibia vara.2
It is classified according to age at onset. Infantile Blount's disease (IBD) or early-onset Blount's disease is recognised as progressive tibial bowing with onset before the age of 4 years.3 Thompson and Carter divided late-onset Blount's disease into juvenile (onset 4-10 years) and adolescent Blount's disease (onset >11 years).4
The disorder is relatively common in South Africa and it is frequently treated by general orthopaedic surgeons. Recurrent deformity and repeat surgery are associated with worse outcome at skeletal maturity.5 The aim of this narrative review is to highlight key principles related to the diagnosis, evaluation, assessment of recurrence risk, and treatment strategy selection for children with IBD.
A literature search on Google Scholar, Medline, LWW, Scopus and ScienceDirect was performed using the keywords 'infantile or early-onset tibia vara', 'infantile or early-onset Blount disease', 'infantile or early-onset Blount's disease'. Retrieved studies and other articles (identified from references and textbooks) were reviewed. Studies relevant to these principles were selected for this narrative review.
Diagnosis
It may be difficult to diagnose the cause of bow-leg deformity between the ages of 1 and 3 years. Lower limb bowing may be apparent, physiological or pathological. Apparent bowing is easily identified as due to an immature gait pattern (hip flexion and external rotation) where knee flexion is perceived as varus due to the rotation out of the plane of gait progression.6 Causes of pathological bowing include Blount's disease, rickets, focal fibrocartilaginous defect (FFCD), skeletal dysplasia, or the result of trauma or infection. Differentiation between causes of pathological bowing is easily made on clinical and radiological evaluation and beyond the scope of this review. Physiological bowing and IBD is often indistinguishable clinically and radiologically before the age of 36 months. IBD is diagnosed when progressive pathological bowing of the proximal tibia is associated with medial metaphyseal lucency as well as sclerosis, fragmentation and delayed ossification of the medial epiphyseal ossific nucleus.7 These classic radiographic features as described by Langenskiöld and Riska appear between 18 and 36 months. Early diagnosis is important as earlier treatment is associated with improved outcomes.5
Several methods should be used to make the diagnosis. As the deformity is diaphyseal in physiological bowing, the clinical 'cover-up' test is useful. This test is performed by obscuring the middle of the tibial diaphysis with the examiner's hand perpendicular to the limb axis, while the other hand holds the ankle with the limb rotated so that the patella is facing directly upward. The alignment of the proximal tibia is assessed in relation to the thigh.6 A positive test results if normal valgus proximal tibial alignment is absent. It has been shown to have a sensitivity of 1.00 (95% CI [0.97, 1.03]), specificity of 0.86 (95% CI [0.75, 0.97]), positive predictive value (PPV) of 0.72 (95% CI [0.52, 0.92]) and negative predictive value (nPV) of 1.0 (95% CI 0.99, 1.01).6 Calculating the likelihood (+) and likelihood (-) ratios from these findings, this test ranks between 'excellent' and 'very good' in terms of impact on likelihood.8 Other findings indicating pathological bowing is unilateral, severe and progressive bowing with the apex of deformity located at the proximal tibial metaphysis. Radiological features of physiological bowing (Figure 1) include diaphyseal bowing of both the femur and tibia, medial distal femoral and proximal tibial metaphyseal beaking, and thickening of the medial cortices of both the femur and tibial diaphysis. Delayed medial ossification of the distal femoral and proximal tibial epiphyseal ossification centres results in a triangular appearance.9 Several radiographic features have been identified to aid in diagnosing IBD. These include the metaphyseal-diaphyseal angle (MDA), epiphyseal-metaphyseal angle (EMA) (Figure 2) and relative contribution to varus from distal femur and proximal tibia.10 Feldman and Schoenecker found that the MDA averaged 9°±3.9° in patients with physiological bowing and 19°±5.7° for patients with Blount's disease. They reported a >5% false positive and false negative rate if the MDA was between 9° and 16° and cautioned against using the MDA in isolation.11 A combination of MDA and EMA, using the cut-off values of >10° and >20° respectively, resulted in positive prediction for IBD with a sensitivity of 1.00 (95% CI [0.80, 1.00] and specificity 0.80 (95% CI [0.68, 0.98]) in a study by Davids et al.12

Park et al. determined the rate of spontaneous correction in a cohort of 174 children with physiological bowing (MDA>9°; mean age 17.3 [12-30 months]) to be 3° at 6 months and 6° at 12 months. As expected, there was no corresponding spontaneous correction of MDA in 32 children with IBD.13 Consideration of all these factors should inform the management strategy. Close observation (clinical and X-ray) in borderline cases is essential to avoid delayed surgical treatment of children with IBD.
Aetiology and pathogenesis
The cause of Blount's disease is likely to be multifactorial.14 Several factors have been implicated and investigated. These include a mechanical aetiology (due to its association with obesity and reported early walking), as well as genetic factors. Aetiological factors in Blount's disease are often investigated and reported for mixed cohorts of IBD, juvenile and adolescent Blount's disease.14
Obesity has been associated with Blount's disease since the original reports by Blount and Langenskiöld. Heuter-Volkmann law dictates that compressive forces over the growth plate will inhibit growth while the Delpech law states that tensile forces will result in a growth increase.15 Cook et al. using finite element analysis of the proximal tibia, demonstrated that 30° varus corresponds with a seven-fold increase in compressive forces on the medial side of the proximal tibial growth plate. Lateral tensile forces were similarly increased above normal. Single leg stance in a 5-year old obese child with 10° varus resulted in compressive forces sufficient to inhibit growth. In a 5-year old with 10° varus and normal weight, these forces (while resulting in a two-fold increase compared to normal alignment) were not sufficient to inhibit growth.16
Scott et al. found that body mass index (BMI) and BMI percentile were significantly (p<0.001 and p=0.003 respectively) associated with a diagnosis of IBD in a cohort of 69 children (between 2 and 4 years of age) who presented with idiopathic bow legs. (When combined with an MDA cut-off of >10°, BMI>22 kg/m2 had a sensitivity of 0.95, specificity of 1.00, PPV of 1.00 and NPV of 0.98.)17 Dietz et al. found a significant correlation (r=0.75, p<0.01) between the magnitude of varus (as measured by the femoral-tibial shaft angle) and body weight in children with Blount's disease.18 Similarly, Sabharwal et al. found a significant correlation (r=0.74, p<0.0001) between mechanical axis deviation (MAD) and BMI in IBD.19 This clear association between obesity and Blount's disease does, however, not explain why the disorder also occurs in children with normal weight (often with severe deformity) or occurs unilaterally in approximately 50% of cases.
Early walking has been proposed as a risk factor associated with IBD.10 In contrast to obesity, no robust evidence exists to support this. On the contrary, Bathfield found normal (average 13 months) age of walking in a cohort of 110 children with Blount's disease.20 More recently, Mehtar et al. confirmed this finding in a cohort of 108 children with Blount's disease from the same area (Johannesburg, South Africa).21
Vitamin D plays multiple roles in the musculoskeletal system, and its association with Blount's disease has been investigated. One study in a cohort of patients attending an obesity clinic found 12 children with Blount's disease, and an association with low levels of vitamin D.22 This finding was referenced in recent literature reviews.23,24 In a well-designed South African study, Lisenda et al. contradicted this by reporting the prevalence of Vitamin D deficiency in a cohort of 50 children with Blount's disease to be similar to that of healthy children living in the same area.25
It is clear that the aetiology of Blount's disease is multifactorial. Further research into environmental, nutritional and genetic factors is needed to identify at-risk children, as well as potentially modifiable factors.
Clinical assessment
Patient profile
IBD is defined as pathological genu varum with onset before the age of 4 years. In the South African context, children may present late with severe deformity. A thorough history is important to determine age at onset of deformity. Based on meta-analysis, children with IBD are more likely to have bilateral disease (53% OR=4.30 95% CI [2.27-8.17], p<0.0001) and less likely to be male (61% OR=0.32 95% CI [0.13-0.78], p=0.01) when compared to adolescent Blount's disease.26 Mehtar et al. confirmed these findings with 82% being female and 64% having bilateral disease from a cohort of 108 children with Blount's disease (44 with IBD).21
Measuring and plotting the BMI percentile for age and sex is essential to identify children at risk for obesity and to initiate early intervention.
Deformity
Clinical assessment of the extent of proximal tibial varus, internal tibial torsion and procurvatum is essential. Varus instability at 15° of knee flexion (Siffert-Katz sign) is the first sign of intra-articular deformity due to posteromedial depression of the tibial plateau.27 Late presentation results in significant varus instability during the stance phase of gait as well as during full extension when the knee is examined in the supine position.
The lower limb rotational profile is assessed by the method described by Staheli et al.28Internal tibial torsion is universally present, but internal femoral torsion may also contribute to in-toeing, as described by Aird et al.29
A careful assessment of proximal tibial procurvatum should be made ensuring the patella is facing forward. This component of the deformity may be underestimated and should be correlated with X-ray measurements.23
Imaging
Plain X-ray
Standardised standing long films including the hip and ankle should be obtained in the anteroposterior (AP) and lateral (LAT) plane. Several authors have emphasised the importance of ensuring the patella is facing forward for coronal plane deformity analysis.30,31 A common error is placing the limb with the foot facing forward. This leads to external rotation of the knee due to internal tibial torsion. Procurvatum in this position appears as increased varus alignment. Overlap at the proximal medial tibia obscures radiographic features and prevents accurate Langenskiöld staging. In addition, rotation will result in incorrect assessment of the tibiofemoral angle (TFA) and MDA, making the differentiation between physiological bowing and IBD difficult. In order to ensure analysis is accurate (as the patella may not yet be ossified), it is critical to ensure that no more than 60% of the proximal fibula should overlap with the proximal tibia on the AP X-ray.30
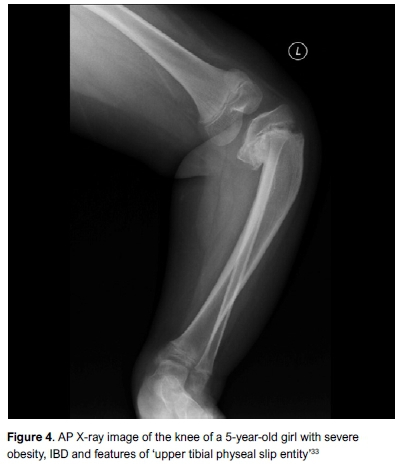
The classic X-ray features of IBD were described in detail by Langenskiöld as progressive changes during skeletal maturation (Figure 3 and Table I). Lateral physeal widening in association with Blount's disease has also been described.32 In what could be seen as a progression of this, Sanghrajka et al. described the 'slipped upper tibial epiphysis' in three children with IBD. This entity consists of a dome-shaped metaphysis, open physis with dissociation of the lateral borders of the epiphysis and metaphysis, with infero-medial epiphyseal displacement (Figure 4). It may be associated with severe obesity.33 These features were not described by the Langenskiöld classification and may have implications for management.

Multi-planar deformity analysis of long leg films is essential to ensure comprehensive deformity correction. Distal femoral frontal plane deformity has been described in association with IBD by several authors. Standing standardised long leg films provide accurate data for pre-operative planning. Gordon et al. showed that the distal femur is either normal or has mild varus deformity.34
Others have noted compensatory distal femoral valgus associated with late presentation.24 Firth et al. demonstrated in a comparative study between infantile, juvenile and adolescent Blount's disease that distal femoral varus may be present in all groups.35 A limitation of this study was that long leg films were not available for all cases. In a detailed description of multi-planar deformity analysis with long leg films, Sabharwal et al. found no significant difference in distal femur, proximal femur or distal tibial frontal plane alignment in either IBD or late-onset Blount's disease.36 Evaluation of these studies reveals that, while the average distal femoral alignment may be normal to slight varus, a wide range of distal femoral deformity exists. Distal femoral alignment should be assessed in each individual case. Sabharwal et al. also emphasised the importance of the sagittal plane. They showed that proximal tibial procurvatum is routinely underestimated clinically and X-ray assessment is essential.36
The aim of X-ray classification in IBD is to inform prognosis (to prevent recurrence the first step is to identify the child at risk), guide management and categorise patients reliably to facilitate communication, records and research. No such classification exists for Blount's disease.
Early stages of the Langenskiöld classification have been shown to not differentiate between physiological bowing and IBD.37 Recurrence has been associated with either Langenskiöld stage >338 or >4.39 Unfortunately, reliability and reproducibility of the intermediate stages of the Langenskiöld classification have been brought into doubt,40 limiting its use to predict recurrence. While more recently Erkus et al. showed excellent inter- and intra-observer reliability, separate assessment of the intermediate grades was not done.41 In contrast, Du Plessis et al. from Johannesburg showed only fair reliability (k=0.24) and reproducibility (k=0.38) and cautioned against its use for prognostication, management planning and research.42
LaMont et al. simplified the Langenskiöld classification into three grades in an attempt to better predict the risk of recurrence. Their classification is based on the morphology of the metaphyseal/ epiphyseal slope. Langenskiöld stages 4, 5, 6 and some stage 3 fall into group C. They described a recurrence rate of 22.5%, 20.7% and 71.7% in groups A, B and C respectively.43
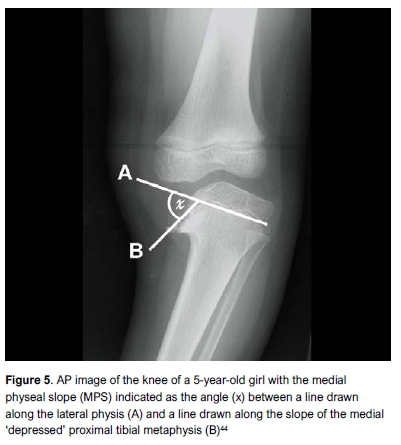
Kling et al. measured the medial physeal slope (MPS) (Figure 5) and demonstrated an association with recurrence when this is greater than 60°.44 Kaewpornsawan et al. confirmed an MPS angle >60° to be the most significant factor associated with recurrence by using multivariate logistic regression analysis.45 In a paper presented at the Combined Meeting of the Orthopaedic Associations in 2016, Maré et al. showed in a group of 20 children with 35 limbs that an MPS>60° was a highly significant predictor of recurrence (OR=1.4 95% CI [1.11-1.82], p=0.005) with a sensitivity of 0.79, specificity of 0.95, PPV of 0.92 and NPV of 0.87.46
Arthrogram
Ossification of the medial proximal tibia is delayed in younger children with IBD. Subsequently the extent of the depression of the medial tibial plateau may be overestimated on plain X-ray (Figure 6). Arthrogram is useful intra-operatively to accurately determine the extent of depression of the medial plateau as well as to confirm adequate elevation.

Magnetic resonance imaging (MRI)
The first MRI study focused on the intra-articular features of Blount's disease confirmed the depression of the medial plateau as well as delayed ossification of the cartilaginous epiphysis.47 An abnormally large medial meniscus with abnormal signal intensity is also a key feature.48 Other features include cartilage intrusion into the medial metaphysis, widening and depression of the medial physis, widening of the lateral physis and osteochondral injury of the medial femoral condyle.49 Mukai et al. attempted to differentiate between physiological bowing and IBD with MRI investigation of 13 children at an average age of 18 months.50 They demonstrated high signal on T2-weighted images in all cases in the epiphysis, but also in the physis and perichondral region of those more likely to develop IBD (as determined by MDA). MRI investigation is not routine practice for the assessment of IBD disease due to availability, expense and possible need for sedation.24 Further research is required to define its role in the diagnosis and assessment of IBD.
Management
Bracing
Several studies have shown promising results, while others have questioned these findings due to methodology, and the difficulty in differentiating physiological bowing from IBD in this young age group.10 Doubt exists about its ability to alter the natural course of IBD.24 Children with physiological genu varum will improve without treatment. Bracing is therefore unnecessary, difficult and expensive. Bracing a child with confirmed IBD may result in a delay in obtaining surgical deformity correction, which will predispose to recurrent deformity. Further research is required to evaluate the role of bracing on the natural history of IBD.
Proximal tibial osteotomy and acute correction
Once the diagnosis of IBD is confirmed, corrective proximal tibial osteotomy before the age of 4 years decreases the risk of recurrent deformity.10 Several techniques have been described, including the dome (curved proximal tibial osteotomy first described by Langenskiöld in 1929)3, spike, oblique (described by Rab)51, serrated W/M osteotomy,52 transverse, open or closing wedge osteotomies.10 No superiority of one technique over another has been demonstrated. When performed early, the aim is to overcorrect varus to 5-10° of valgus2,3,53 while simultaneously correcting procurvatum and internal tibial torsion.
The corrected position may be maintained by casting, internal fixation, a combination of the two, or by external fixation.10 The complication rate of tibial osteotomies is low.54 Incision placement is important as recurrence rate is high. An oblique incision from proximal medial to distal lateral is ideal, as it facilitates easy exposure of the medial tibial condyle through the same scar should elevation be required in the future. Possible complications include transient or permanent peroneal nerve palsy, pin-track infection, delayed union, over- or under-correction, compartment syndrome or vascular injury. Prophylactic anterior compartment fasciotomy and drain insertion is strongly recommended.10 Normal growth following realignment is re-established in the proximal tibia in only 50-60% of cases.3,5,38,53,55 If growth resumes symmetrically, no further treatment is necessary. If normal growth does not resume, the deformity will recur, which is associated with worse outcome.5,39
Risk factors for recurrence following proximal tibial osteotomy
Age at osteotomy
Langenskiöld and Riska reported that if osteotomy and deformity correction is performed before the age of 8 years, recurrence is unlikely.3 This has not been the experience of most other authors. Ferriter and Shapiro found the watershed age to be 4 years and 6 months,38 while Schoenecker et al. reported the age to be 5 years.56 Kaewpornsawan et al. recommended surgery before the age of 3 years to prevent recurrence.45 Thompson et al. in a study on 58 South African children (100 limbs) found that age >4 years at treatment (p<0.001) is significantly associated with recurrence.55
Obesity
Ferriter and Shapiro showed an increased risk of recurrence in children with BMI>97th centile.38 Thompson et al. confirmed the association between BMI>95th centile and recurrence (p=0.007).55
Langenskiöld stage
Higher Langenskiöld stage is associated with increased risk of recurrence. Ferriter and Shapiro reported that all children with stage 5 and 6 recurred, with an increase in recurrence rate from stage 2 to 4.38 Doyle et al. found that all cases with Langenskiöld 4 X-ray appearance recurred.5
Thompson et al. confirmed significance (p<0.001) of the association between advanced Langenskiöld stage and recurrence risk.55 The poor inter-observer reliability for intermediate Langenskiöld stages limits the prognostic value of this finding. Ideally a new classification system is required that will accurately stratify patients according to risk for recurrence in order to better guide treatment.
Medial physeal slope (MPS)
The measurement of slope of MPS is a quantifiable assessment of the severity of the distortion of the morphology of the medial proximal tibia in IBD. Several authors have emphasised the significance association of MPS>60° with risk of recurrence following corrective osteotomy.44,45,55
These factors have only been studied as part of retrospective studies of relatively small, diverse patient groups. Further study to elucidate their relative and collective value as part of a predictive scoring system is required.
Measures to decrease risk of recurrence following osteotomy
Correction into physiological valgus alignment
Several authors have recommended correction to physiological valgus alignment.1-3,38,53 While it is clear that under-correction should be avoided,53 several authors have cautioned against excessive valgus correction, as this may not remodel if normal medial growth resumes.1-3 Eamsobhana et al. showed no difference in recurrence between limbs overcorrected to >13° valgus versus those corrected to normal alignment.57
Medial epiphysiolysis
Excision of the posteromedial proximal tibial physis/area of disordered growth has been described as an adjunctive procedure to proximal tibial realignment osteotomy in an attempt to restore symmetrical growth.58 Andrade and Johnstone reported their experience with this procedure in 24 children (27 limbs).59 They reported favourable results in children younger than 7 years, but did not recommend the procedure for older children. These authors noted that complete resection was challenging. Unlike post-traumatic growth arrest, there is no well-defined physeal bar in Blount's disease.10 Further research to identify the role of this additional procedure is required.
Lateral epiphysiodesis
Recurrent deformity is certain when no medial growth potential remains. Langenskiöld and Riska recommended lateral proximal tibial and fibular epiphysiodesis (in addition to proximal tibial and fibula osteotomy) in children older than 9 years when the medial physis was closed.3 Epiphysiodesis may be achieved by percutaneous drilling, open Phemister technique or transphyseal screw fixation.60 The challenge remains to predict which patients have no medial growth potential. An error in this assessment will result in progressive overcorrection, necessitating further surgery (medial epiphysiodesis and deformity correction) and loss of longitudinal growth potential. Further research to improve risk stratification in terms of recurrence is of critical importance to improve outcomes in the treatment of IBD.
Guided growth
Paediatric deformity correction by reversible hemi-epiphysiodesis has several advantages. Stevens reignited enthusiasm for this technique with the publication of his results with the tension-band plate in 65 femoral and tibial deformities.61 Success of guided growth in IBD relies on three variables: medial proximal tibial growth potential, magnitude of deformity, and growth remaining. Scott published an encouraging report of 89% effective correction in 14 children (18 limbs) with IBD treated with tension-band plates.62 The average age at treatment was 4.8 years (2.8- 8.7 years). The BMI percentile was >93rd centile in all but one child. Complications included wound infection and screw breakage. Correction of tibial rotation was noted to occur slower than correction of varus. Persistent in-toeing was associated with increased femoral anteversion. All but one child presented before the age of 5 years and the authors stated that success is likely to be related to early treatment.62
Griswold et al. in 2020 reported on 11 children (17 limbs) with IBD treated with tension-band plates.63 Cases were divided into two groups according to Langenskiöld stage (stage <2 or >3). The median ages were 3.2 years (±1.4) and 4.36 years (±2.16) for the groups with Langenskiöld stage <2 and >3 respectively. They reported a 100% correction rate in children with Langenskiöld stage <2 and a 33% recurrent deformity rate (treated with repeat tension-band plating). This correction rate fell to 40% in children with Langenskiöld stage >3 with a 100% recurrence or incomplete correction rate. Complications included surgical site infection, screw breakage and overcorrection. The authors cautioned against the use of tension-band plating in children with Langenskiöld stage >3 IBD.63
Stevens noted anecdotally that rotational deformity may correct together with coronal plane deformity during treatment with tension-band plating.64 Cobanoglu et al., in an animal study, showed that rotational long bone deformity could be corrected with oblique placement of a tension-band plate.65 Further study is required to determine the efficacy of guided growth to correct internal tibial torsion in IBD. Adherence to follow-up appointments is essential to monitor for both overcorrection due to delayed removal and recurrent deformity as a result of rebound growth after removal. As age at deformity correction through osteotomy is a critical factor associated with recurrence,10 and evidence from studies on guided growth confirms an association between success and earlier treatment, guided growth should be used with caution in children with IBD older than 4 years or Langenskiöld stage >3.
The role of guided growth in children with IBD is yet to be defined. Factors associated with risk of failure should be investigated. Well-designed randomised studies comparing guided growth and standard deformity correction techniques are needed.
Gradual correction with external fixation
Excellent correction can be achieved with external fixator-assisted gradual deformity correction. A major advantage is that the tibia may be lengthened at the same time as multi-planar deformity correction. The Ilizarov fine-wire circular fixator has been shown to be a safe and effective technique to correct deformity while allowing early mobilisation and weight bearing.66 Computer-assisted hexapod circular fixators utilise a 'virtual hinge,' thereby allowing all aspects of the deformity to be corrected with a single frame. This allows for complex deformity correction, especially when previous surgery for severe deformity resulted in mechanical axis translation. Another significant advantage is the ability to fine-tune deformity correction without the need to return to the operating room. Several authors reported safe and effective deformity correction.67,68
A systematic review and meta-analysis analysing acute vs gradual correction (mono-lateral, hexapod as well as Ilizarov external fixators) reported no robust evidence to support superiority in terms of accuracy of correction or complication rates. The studies analysed were mostly small, retrospective cases series with heterogenous techniques and reporting. The complication rate of either technique was low.69 Feldman et al. specifically compared computer-assisted hexapod deformity correction with acute correction. They demonstrated better correction of sagittal plane deformity and axis translation with hexapod-assisted deformity correction.70
Hexapod-assisted deformity correction has specific advantage in late-presenting or recurrent IBD or where severe obesity complicates fixation, especially in unilateral cases where leg-length discrepancy can be corrected at the same time. Where significant joint instability exists due to medial plateau depression, elevation can be performed at the same time. Several authors reported safety and efficacy of this technique based on small, retrospective case series.71-73 Larger series are required to confirm a low complication rate. Pin-track infection is a common complication of fine-wire circular fixator-assisted deformity correction. This is especially common on the tension side (medially) on the proximal tibia. Major pin-track infection could theoretically result in deep infection at the elevation site, catastrophically resulting in osteomyelitis and septic arthritis (if joint penetration inadvertently occurred at the time of elevation). Staged elevation and subsequent gradual deformity correction may be a safer alternative.
Neglected infantile Blount's disease
Medial elevation combined with lateral epiphysiodesis
If IBD is not identified and treated early, medial articular depression can occur (Figure 6), together with worsening proximal tibial varus, procurvatum and internal rotation.74 This defines neglected IBD. Blount recognised knee instability in three cases that was responsible for part of the varus deformity.1 Langenskiöld attributed this to the sloping of the medial tibial condyle in neglected cases.3 Siffert and Katz confirmed the presence of the depression of the medial tibial plateau with direct visualisation at arthrotomy and described the pathoanatomy of this deformity in detail. They advocated elevation of the medial tibial plateau as an essential part of treatment.27 Medial articular elevation is normally combined with lateral epiphysiodesis of the proximal tibia and fibular and metaphyseal proximal tibial osteotomy to correct residual varus, procurvatum and internal rotation. Van Huyssteen et al. emphasised the importance of concomitant lateral proximal tibial epiphysiodesis at the same time as medial joint line elevation to prevent recurrence.74
The existence of medial articular depression has been brought into question in a small study of ten children (17 limbs) with arthrogram and MRI investigation. These authors speculated that delayed ossification of the medial proximal tibial structures were responsible for the appearance of depression.75 Other authors, by direct visualisation, arthrogram and MRI investigation, have since confirmed the presence of true posteromedial depression in addition to delayed ossification of the medial structures in neglected IBD cases.74,76 The age from which medial elevation and lateral epiphysiodesis is indicated is controversial. Langenskiöld originally suggested 9 years when medial growth arrest and articular depression is confirmed.9 Currently, medial elevation is indicated for children older than 6 years with IBD, Langenskiöld stage 5 or 6 and significant medial articular depression.10,74 Controversy hinges on concern for loss of growth potential if the procedure is performed before medial proximal tibial growth arrest is confirmed. Further research is essential to improve our ability to predict medial growth potential, as well as the long-term outcome of the surgical management of neglected IBD.
Concomitant femoral deformity correction
Significant distal femoral frontal plane malalignment will result in joint line obliquity if the mechanical axis is restored through proximal tibial deformity correction alone. Schoenecker et al. were the first to report on the surgical treatment of distal femur in IBD. They performed concomitant corrective osteotomy for >10° distal femoral valgus in four cases.77
The long-term effect of joint line obliquity is uncertain. No robust evidence exists to predict when concomitant distal femoral and proximal tibial deformity correction is indicated to improve outcome. Further research is also required to assess the incidence of symptomatic femoral rotational deformity in IBD and to determine the threshold for surgical correction.
Long-term outcome
Several authors have reported on the long-term outcome after treatment of IBD. Ingvarsson et al. found 11/89 knees with signs of arthrosis.78 Zayer found a much higher rate of arthritis (11/27 knees in patients older than 30 years).79 Doyle et al. followed 17 children to skeletal maturity. They found 9/28 knees to be symptomatic at a mean age of 20 years. Symptomatic knees all demonstrated ligamentous, meniscal or bony pathology on MRI and arthroscopy. There was a significant association between repeat osteotomy and a symptomatic knee at skeletal maturity.5 Hofmann et al. followed 12 children (19 limbs) to skeletal maturity. Symptomatic knees (12/19) had degenerative changes at arthroscopy or arthrotomy.39 Both Doyle and Hofmann recommended early osteotomy before permanent physeal damage occurred on the basis of their findings.
Total knee replacement after Blount's disease may be complex. Natoli et al. recommended that surgeons be prepared to address posteromedial tibial bone defects and consider constrained arthroplasty at the index procedure.80
Conclusion and future direction
Decision-making in IBD is complex. Discriminating between IBD and physiological bowing may be difficult. Meticulous clinical and X-ray follow-up is essential to prevent a delay in corrective osteotomy beyond 4 years if IBD is confirmed. Once a child presents with neglected IBD or recurrent deformity, it is essential to identify all aspects of the deformity to ensure comprehensive correction and prevent recurrence by lateral epiphysiodesis.
Future research should focus on developing risk stratification tools to predict medial growth potential. The role of medial epiphysiolysis and guided growth requires further definition. Treatment strategies should aim to achieve lasting deformity correction by decreasing recurrence risk while avoiding unnecessary growth ablation.
Follow-up until skeletal maturity and beyond is essential to optimise outcome and inform our understanding of the prognosis in IBD.
Ethics statement
The author/s declare that this submission is in accordance ethical guidelines. Institutional Review Board (IRB) ethical approval was not required as this was a literature review.
Declaration
The authors declare authorship of this article and that they have followed sound scientific research practice. This research is original and does not transgress plagiarism policies.
Author contributions
DMT contributed to the conceptualisation of the article and performed manuscript review. PHM performed the literature review, contributed to the conceptualisation, preparation and revision of the manuscript.
ORCID
Maré PH https://orcid.org/0000-0003-1599-7651
Thompson DM https://orcid.org/0000-0003-2607-3999
References
1. Blount WP. Tibia vara. Osteochondrosis deformans tibiae. J Bone Joint Surg. 1937;19:1-29. [ Links ]
2. Langenskiöld A. Tibia vara (osteochondrosis deformans tibiae). A survey of twenty-three cases. Acta Chir Scand. 1952;103:1-22. [ Links ]
3. Langenskiöld A, Riska EB. Tibia vara (osteochondrosis deformans tibiae) A survey of seventy-one cases. J Bone Joint Surg (Am). 1964;46:1405-20. [ Links ]
4. Thompson GH, Carter JR. Late-onset tibia vara (Blount's disease). Current concepts. Clin Orthop Relat Res. 1990;255:24-35. [ Links ]
5. Doyle BS, Volk AG, Smith CF. Infantile Blount disease. Long-term follow-up of surgically treated patients at skeletal maturity. J Pediatr Orthop. 1996;16(4):469-76. [ Links ]
6. Davids JR, Blackhurst DW, Allen BL Jr. Clinical evaluation of bow legs in children. J Pediatr Orthop B. 2000;9:278-84. [ Links ]
7. Levine AM, Drennan JC. Physiological bowing and tibia vara. The metaphyseal-diaphyseal angle in the measurement of bowleg deformities. J Bone Joint Surg (Am). 1982;64:1158-63. [ Links ]
8. WHO recommendations on the diagnosis of HIV infection in infants and children. Annex 4: Characteristics of a screening test. World Health Organization; 2010. [ Links ]
9. Zayer M. Long-term results after physiological genu varum. J Pediatr Orthop B. 2000;9:271-77. [ Links ]
10. Sabharwal S. Blount disease. Current concepts review. J Bone Joint Surg (Am). 2009;91-A:1758-76. [ Links ]
11. Feldman MD, Schoenecker PL. Use of the metaphyseal-diaphyseal angle in the evaluation of bowed legs. J Bone Joint Surg (Am). 1993;75-A:1602-609. [ Links ]
12. Davids JR, Blackhurst DW, Allen BL Jr. Radiographic evaluation of bowed legs in children. J Pediatr Orthop. 2001;21:257-63. [ Links ]
13. Park BK, Park KB, Kwak YH, et al. A comparative evaluation of tibial metaphyseal-diaphyseal angle changes between physiologic bowing and Blount disease. Medicine. 2019;98(17):1-6. [ Links ]
14. Banwarie RR, Hollman F, Meijs N, et al. Insight into the possible aetiologies of Blount's disease: a systematic review of the literature. J Pediatr Orthop B. 2020 Jul;29(4):323-36. [ Links ]
15. Gettys FK, Jackson JB, Frick SL. Obesity in pediatric orthopaedics. Orthop Clin N Am. 2011;42:95-105. [ Links ]
16. Cook SD, Lavernia CJ, Burke SW, Skinner HB, Haddad RJ Jr. A biomechanical analysis of the etiology of tibia vara. J Pediatr Orthop. 1983;3:449-54. [ Links ]
17. Scott AC, Kelly CH, Sullivan E. Body mass index as a prognostic factor in development of infantile Blount disease. J Pediatr Orthop. 2007;27:921-25. [ Links ]
18. Dietz WH, Gross WL, Kirkpatrick JA Jr. Blount disease (tibia vara): another skeletal disorder associated with childhood obesity. J Pediatr. 1982;101(5):735-37. [ Links ]
19. Sabharwal S, Zhao C, McClemens E. Correlation of body mass index and radiographic deformities in children with Blount disease. J Bone Joint Surg. 2007;89:1275-83. [ Links ]
20. Bathfield CA, Beighton PH. Blount disease. A review of etiological factors in 110 patients. Clin Orthop Relat Res. 1978;135:29-33. [ Links ]
21. Mehtar M, Ramguthy Y, Firth GB. Profile of patients with Blount's disease at an academic hospital. SA Orthop J. 2019;18(3):30-35. [ Links ]
22. Montgomery CO, Young KL, Austen M, et al. Increased risk of Blount disease in obese children and adolescents with vitamin D deficiency. J Pediatr Orthop. 2010;30(8):879-82. [ Links ]
23. Sabharwal S. Blount disease: An update. Orthop Clin N Am. 2015;46:37-47. [ Links ]
24. Birch JG. Blount disease. J Am Acad Orthop Surg. 2013;21:408-18. [ Links ]
25. Lisenda L, Simmons D, Firth GB, et al. Vitamin D status in Blount disease. J Pediatr Orthop. 2016;36:e59-e62. [ Links ]
26. Rivero SM, Zhao C, Sabharwal S. Are patient demographics different for early-onset and late-onset Blount disease? Results based on meta-analysis. J Pediatr Orthop. 2015;24(6):515-20. [ Links ]
27. Siffert RS, Katz, JF. The intra-articular deformity in osteochondrosis deformans tibiae. J Bone Joint Surg (Am). 1970;52-A(4):800-804. [ Links ]
28. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. J Bone Joint Surg (Am). 1985;67-A(1):39-47. [ Links ]
29. Aird JJ, Hogg A, Rollinson P. Femoral torsion in patients with Blount's disease. A previously unrecognised component. J Bone Joint Surg (Br). 2009;91-B:1388-93. [ Links ]
30. Stricker SJ, Faustgen JP. Radiographic measurement of bowleg deformity: variability due to method and limb rotation. J Pediatr Orthop. 1994;14:147-51. [ Links ]
31. Jamali AA, Meehan JP, Moroski NM, et al. Do small changes in rotation affect measurements of lower extremity limb alignment? J Orthop Surg Res. 2017;12:77. [ Links ]
32. Currarino G, Kirks DR. Lateral widening of epiphyseal plates in knees of children with bowed legs. Am J Roentgenol. 1977;129:309-12. [ Links ]
33. Sanghrajka AP, Hill RA, Murnaghan CF, Simpson AHRW, Bellemore MC. Slipped upper tibial epiphysis in infantile tibia vara. J Bone Joint Surg (Br). 2012;94-B:1288-91. [ Links ]
34. Gordon JE, King DJ, Luhmann SJ, Dobbs MB, Schoenecker PL. Femoral deformity in tibia vara. J Bone and Joint Surg (Am). 2006;88-A:380-86. [ Links ]
35. Firth GB, Ngcakani A, Ramguthy Y, Izu A, Robertson A. The femoral deformity in Blount's disease: a comparative study of infantile, juvenile and adolescent Blount's disease. J Pediatr Orthop B. 2020;29(4):317-22. [ Links ]
36. Sabharwal S, Lee J Jr., Zhao C. Multiplanar deformity analysis of untreated Blount disease. J Pediatr Orthop. 2007;27:260-65. [ Links ]
37. Shinohara Y, Kamegaya M, Kuniyoshi K, Moriya H. Natural history of infantile tibia vara. J Bone Joint Surg (Br). 2002;84-B:263-68. [ Links ]
38. Ferriter P, Shapiro F. Infantile tibia vara: Factors affecting outcome following proximal tibial osteotomy. J Pediatr Orthop. 1987;7:1-7. [ Links ]
39. Hofmann A, Jones RE, Herring JA. Blount's disease after skeletal maturity. J Bone Joint Surg. 1982;64-A:1004-1009. [ Links ]
40. Stricker SJ, Edwards PM, Tidwell MA. Langenskiöld classification of tibia vara: an assessment of interobserver variability. J Pediatr Orthop. 1994;14:152-55. [ Links ]
41. Erkus S, Turgut A, Kalenderer O. Langenskiöld classification for Blount disease: Is it reliable? Indian J Orthop. 2019;53(5):662-64. [ Links ]
42. Du Plessis J, Firth GB, Robertson A. Assessment of the reliability and reproducibility of the Langenskiöld classification in Blount's disease. J Pediatr Orthop B. 2020 Jul;29(4):311-16. [ Links ]
43. LaMont LE, McIntosh AL, Jo CH, Birch JG, Johnston CE. Recurrence after surgical intervention for infantile tibia vara: assessment of a new modified classification. J Pediatr Orthop. 2019;39(2):65-70. [ Links ]
44. Kling TF, Volk AG, Dias L, Morgan RC, DeRosa GP. Infantile Blount's disease treated with osteotomy followed to maturity. Orthopaedic Transactions. 1990;14:634-35. [ Links ]
45. Kaewpornsawan K, Tangsataporn S, Jatunarapit R. Early proximal tibial valgus osteotomy as a very important prognostic factor in Thai children with infantile tibia vara. J Med Assoc Thai. 2005;88 Suppl 5:S72-79. [ Links ]
46. Maré PH, Thompson DM, Marais LC, Sartorius B. Evaluating factors predictive of recurrence after proximal tibial osteotomy in early-onset Blount's disease treated before the age of seven years. Combined Orthopaedic Meeting of the Orthopaedic Associations 11-15 April 2016; Paper 393: Abstract book page B303. [ Links ]
47. Ducou le Pointe H, Mousselard H, Rudelli A, Montagne JP, Filipe G. Blount's disease: magnetic resonance imaging. Pediatr Radiol. 1995;25:12-14. [ Links ]
48. Sabharwal S, Wenokor C, Mehta A, Zhao C. Intra-articular morphology of the knee joint in children with Blount disease. A case control study using MRI. J Bone Joint Surg (Am). 2012;94:883-90. [ Links ]
49. Craig JG, Van Holsbeeck M, Zaltz I. The utility of MR in assessing Blount disease. Skeletal Radiol. 2002;31:208-13. [ Links ]
50. Mukai S, Suzuki S, Seto Y, Kashiwagi N, Hwang E. Early characteristic findings in bowleg deformities: evaluation using magnetic resonance imaging. J Pediatr Orthop. 2000;20:611-15. [ Links ]
51. Rab GT. Oblique tibial osteotomy for Blount's disease (tibia vara). J Pediatr Orthop. 1988;8:715-20. [ Links ]
52. Hayek S, Segev E, Ezra E, et al. Serrated W/M osteotomy. Results using a new technique for the correction of infantile tibia vara. J Bone Joint Surg (Br). 2000;82-B(7):1026-29. [ Links ]
53. Chotigavanichaya C, Salinas G, Green P, Moseley CF, Otsuka NY. Recurrence of varus deformity deformity after proximal tibial osteotomy in Blount disease: long-term follow-up. J Pediatr Orthop. 2002;22:638-41. [ Links ]
54. Payman KR, Patenall V, Borden P, Green T, Otsuka NY. Complications of tibial ostoetomies in children with comorbidities. J Pediatr Orthop. 2002;22:642-44. [ Links ]
55. Thompson D, Maré P, Barciela M. A preliminary report on early onset Blount's disease in the KZN Midlands. Orthopaedic Proceedings. 2014;96-B Suppl19:12. [ Links ]
56. Schoenecker PL, Meade WC, Pierron RL, Sheridan JJ, Capelli AM. Blount's disease: a retrospective review and recommendations for treatment. J Pediatr Orthop. 1985;5(2):181-86. [ Links ]
57. Eamsobhana P, Kaewpornsawan K, Yusuwan K. Do we need to do overcorrection in Blounts disease? Int Orthop. 2014;38(8):1661-64. [ Links ]
58. Beck CL, Burke SW, Roberts JM, Johnstone CE II. Physeal bridge resection in infantile Blount disease. J Pediatr Orthop. 1987;7:161-63. [ Links ]
59. Andrade N, Johnston CE. Medial epiphysiolysis in severe infantile tibia vara. J Pediatr Orthop. 2006;26(5):652-58. [ Links ]
60. Campens C, Mousny M, Docquier PL. Comparison of three surgical epiphysiodesis techniques for the treatment of lower limb length discrepancy. Acta Orthop Belg. 2010;76(2):226-32. [ Links ]
61. Stevens PM. Guided growth for angular correction: a preliminary series using a tension band plate. J Pediatr Orthop. 2007;27:253-59. [ Links ]
62. Scott AC. Treatment of infantile Blount disease with lateral tension band plating. J Pediatr Orthop. 2012;32:29-34. [ Links ]
63. Griswold BG, Shaw KA, Houston H, Bertrand S, Cearley D. Guided growth for the treatment of infantile Blount's disease: Is it a viable option? J Orthop. 2020;20:41-45. [ Links ]
64. Stevens PM. Guided growth for deformity correction. Oper Tech Orthop. 2011;21:197-202. [ Links ]
65. Cobanoglu M, Cullu E, Kilimci FS, Ocal MK, Yaygingul R. Rotational deformities of the long bones can be corrected with rotationally guided growth during the growth phase: A study in rabbits. Acta Orthop. 2016;87(3):301-305. [ Links ]
66. Alekberov C, Shevtsov VI, Karatosun V, Günal I, Alici E. Treatment of tibia vara by the Ilizarov method. Clin Orthop Relat Res. 2003;409:199-208. [ Links ]
67. Feldman DS, Madan S, Koval KJ, et al. Correction of tibia vara with six-axis deformity analysis and the Taylor Spatial Frame. J Pediatr Orthop. 2003;23:387-91. [ Links ]
68. Maré P, Thompson DM. The use of gradual correction with the TLHex external fixator in Blount's disease. Orthopaedic Proceedings. 2014;96-B Suppl 19:11. [ Links ]
69. Gilbody J, Thomas G, Ho K. Acute versus gradual correction of idiopathic tibia vara in children. A systematic review. J Pediatr Orthop. 2009;29:110-14. [ Links ]
70. Feldman DS, Madan S, Ruchelsman DE, Sala DA, Lehman WB. Accuracy of correction of tibia vara. Acute vs gradual correction. J Pediatr Orthop. 2006;26:794-98. [ Links ]
71. Hefny H, Shalaby H, El-kawy S, Thakeb M, Elmoatasem E. A new double elevating osteotomy in management of severe neglected infantile tibia vara. J Pediatr Orthop. 2006;26:233-37. [ Links ]
72. Bar-On E, Weigl DM, Becker T, Katz K. Treatment of severe early onset Blount's disease by an intra-articular and a metaphyseal osteotomy using the Taylor Spatial Frame. J Child Orthop. 2008;2:457-61. [ Links ]
73. Fitoussi F, Ilharreborde B, Lefevre Y, et al. Fixator-assisted medial tibial plateau elevation to treat severe Blount's disease: Outcomes at maturity. Orthop & Traumatol: Surg & Res. 2011;97:172-78. [ Links ]
74. Van Huyssteen AL, Hastings CJ, Olesak M, Hoffman EB. Double-elevating osteotomy for late-presenting Infantile Blount's disease. J Bone Joint Surg (Br). 2005;87-B:710-15. [ Links ]
75. Stanitski D, Stanitski CL, Trumble S. Depression of the medial tibial plateau in early-onset Blount disease: myth or reality? J Pediatr Orthop. 1999; 19(2):265-69. [ Links ]
76. Ho-Fung V, Jaimes C, Delgado J, Davidson RS, Jaramillo D. MRI evaluation of the knee in children with infantile Blount's disease: tibial and extra-tibial findings. Pediatr Radiol. 2013;43:1316-26. [ Links ]
77. Schoenecker PL, Johnston R, Rich MM, Capelli AM. Elevation of the medial plateau of the tibia in the treatment of Blount disease. J Bone Joint Surg (Am). 1992;74:351-58. [ Links ]
78. Ingvarsson T, Hägglund G, Ramgren B, Jonsson K, Zayer Ml. Long-term results after infantile Blount's disease. J Pediatr Orthop B. 1998;7(3):226-29. [ Links ]
79. Zayer M. Osteoarthritis following Blount's disease. Int Orthop. 1980;4:63-66. [ Links ]
80. Natoli RM, Nypaver CM, Schiff AP, Hopkinson WJ, Rees HW. Total knee arthroplasty in patients with Blount disease or Blount-like deformity. J Arth. 2016;31(1):124-27. [ Links ]
 Correspondence:
Correspondence:
Dr PH Maré
PO Box 351, Msunduzi, 3231
tel: +27 33 897 3050; cell: +27 83 294 8375; email: phmare@gmail.com
Received: April 2020
Accepted: May 2020
Published: August 2020
Editor: Prof. Nando Ferreira, Stellenbosch University, Cape Town, South Africa
Funding: No funding was secured for this research.
Conflict of interest: PH Maré has received honoraria from Orthofix and Smith & Nephew for teaching and training. DM Thompson has no conflict of interest to declare.













