Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Enology and Viticulture
On-line version ISSN 2224-7904Print version ISSN 0253-939X
S. Afr. J. Enol. Vitic. vol.43 n.2 Stellenbosch 2022
https://doi.org/10.21548/43-2-5161
ARTICLES
Solubility of Quercetin in Wines
Donato LanatiI; Patrizia CascioI; Matteo PollónII; Onofrio CoronaII; Dora MarchiI, *
IEnosis s.r.l., 11043, Fubine (AL), Italy
IIDepartment of Agricultural, Food and Forest Sciences, University of Palermo, Palermo, Italy
ABSTRACT
Quercetin solubility at 18°C and 0°C was determined in a hydroalcoholic buffer solution with a pH of 3.20 and in four Italian wines to study the formation mechanism of quercetin precipitate in wines. The wines selected were Barbera 2018, for its typically high content of bisulphite bleachable pigments, red Cirò 2014, for its typically high content of flavonoids, Sangiovese 2014, for the presence of quercetin deposits in the bottle, and white Cirò 2018, for the absence of red pigments. All the samples were spiked with 30 mg/L quercetin. The amount of quercetin solubilised at 18°C and 0°C in the hydroalcoholic buffer was much lower than in the wines, while that solubilised in Barbera was much higher compared to red Cirò, San-giovese and white Cirò. Solubilised quercetin was lower in all wine samples stored at 0°C than in those stored at 18°C. The pigment composition of the three red wines examined suggests that the over-solubility of quercetin could be due to the formation of soluble co-pigmentation complexes between quercetin and monomer anthocyanins and/or bisulphate-bleachable flavanol-anthocyanin pigments. A positive correlation between quercetin solubility and bleachable pigment was noted: the richer the wine in bleachable pigments, the higher the solubility of quercetin. Quercetin haze formation appeared due to the release of quercetin from co-pigmentation complexes during wine maturation and storage, as its counterpart, antho-cyanins, form non-bleachable pigments or are degraded in hydrolytic or oxidation reactions. Quercetin in aged red wines seems to reach a content similar to that of white wine spiked with quercetin. Nevertheless, the quercetin content of aged red wines in which a quercetin haze has been found could be lower than that when added to white wine, due to its degradation probably being induced by oxidation reactions. Finally, the solubilised quercetin content in white Cirò, which was higher than the hydroalcoholic buffer solution, suggests that there may be different substances in wines than pigments that prevent the growth of quercetin crystals. However, their nature was not determined in this study.
Keywords: Solubility of quercetin, hydroalcoholic buffer solution, red wines, white wines
INTRODUCTION
Quercetin is a phenolic compound which that belongs to the class of flavonols whose glycosides are located in the skins of white and red grapes (Cheynier & Rigaud, 1986; Mat-tivi et al., 2006; Makris et al., 2006; Castillo-Muñoz et al., 2007). Although to a lesser extent, white grapes also contain glycosylated derivatives of kaempferol and myricetin; while those of kaempferol, myricentin, isoramentin, laricitrin and syringetin are found in coloured grapes (Mattivi et al., 2006; Castillo-Muñoz et al., 2007). Quantitatively, quercetin is generally the most important flavonol in red and white grape skins. In some red grapes, however, the content of myricetin-3-glucoside can exceed that of any of the quercetin glyco-sides (Squadrito et al., 2007). Flavonol biosynthesis begins shortly before véraison and can continue until the end of maturation (Keller & Hrazdina, 1998; Downey et al., 2003; Castellarin et al., 2007). It is affected by the level of exposure of the clusters to direct sunlight and could occur in response to and as a defence mechanism against UV-A rays. In shaded bunches it proceeds slowly or to a limited extent (Price et al. 1995; Haselgrove et al., 2000; Downey et al., 2004; Cortell & Kennedy, 2006), while it is promoted in bunches directly exposed to sunlight (Downey et al., 2004).,
In white winemaking, the diffusion of flavonol glyco-sides from grape skin to the must is usually very little for the short contact time between the must and the grape skin. The content of quercetin glycosides is also very poor in must obtained from correctly cryo-macerated crushed white grapes (use of pre-cooled grapes, soft crushing, limited moving of crushed grapes, no SO2). In red winemaking, the flavonols spread from the skin cells to the must during grape crushing and fermentative maceration. The reaction of the hydrolysis of flavonol glycosides with the production of individual aglycons starts at the same time. The rate at which this reaction occurs suggests that it is catalysed by enzymes. However, further studies are needed to elucidate its mechanism. Due to these hydrolytic reactions, quercetin has also been found in young red wines, sometimes reaching a higher level than expected on the basis of its solubility in wine: 3 mg/L to 4 mg/L (Somers & Ziemelis, 1985), around 5 mg/L (Boulton, 2001), and 3 mg/L (Gambuti et al., 2020).
Despite this over-solubility, in the last few years quer-cetin haze has been found in some bottles of aged Sangio-vese red wines after quite long periods since the end of wine maturation (Lanati et al., 2014; Gambuti et al., 2020). It does not deal with a new phenomenon, as quercetin deposits have also been found in a red wine in the past (Ziemelis & Pickering, 1969), as well as in a white one (Somers & Ziemelis, 1985). More recently, quercetin precipitate has been found in wines from some other cultivars and terroirs in both hemispheres (Marchi et al., 2019), probably due to the current changes in climate. Even though it has been proven that the risk of formation of a quercetin haze decreases in wines that have undergone contact with oxygen (micro-oxygenation, maturation in the barrel, racking) (Lanati et al., 2014; Gam-buti et al., 2020) due to the high antioxidant power of this compound, quercetin over-solubility and the mechanism of its precipitation in wines have to be rationalised. In order to clarify these problems, in this work we have studied the influence of the nature and content of wine pigments on the solubility of quercetin in wines of different ages and phenolic composition.
MATERIALS AND METHODS
Standards and reagents
Quercetin, myricetin and β-glucosidase were purchased from Sigma-Aldrich (Milan, Italy); and sodium metabisulphite (Na2S2O5), NaOH, NaCl, citric acid, ortho-phosphoric acid, methanol LiChrosolv and ethanol 96% v/v from Merck (Milan, Italy).
Composition of solutions
Quercetin solution was prepared as follows: 3 g/L of quercetin in methanol. Hydroalcoholic solution was prepared by placing 5 g/L of tartaric acid and 22.2 mL/L of 1 M NaOH in deionised water, and 125 mL/L of 95% to 96% ethanol was added. The final pH of the buffer was adjusted beforehand to reach a final volume with NaOH 1 M or HCl 1 M to 3.2.
Chemical analysis
Determination of flavonols in wine
Quercetin, myricetin and their glucoside and glucuronide derivatives have been determined by HPLC, as described by Lanati et al. (2021); briefly: 10 mL of wine was added to 0.5 g NaCl and extracted three times with 20 mL ethyl acetate. The solvent was evaporated under vacuum and the residue was dissolved in 2 mL of a solution composed of 60% 10-3 M H3PO4 in water and 40% methanol. Finally, the sample was filtered through a 0.45 μm membrane filter and injected for HPLC.
A Perkin-Elmer HPLC-DAD model Series 200 was used, equipped with a guard column LiChrospher® 100 RP-18 (5 μm) (LiChroCart® 4-4, Merck, Milan, Italy) and a column LiChrospher® 100 RP-18 (5 μm) (LiChroCart® 250-4, Merck, Milan, Italy).
Two solvents were used for the chromatographic elution: H3PO4 10-3 M in H2O (solvent A); and methanol (solvent B). The gradient of analysis for the latter solvent was: 0% for 0 min, 5% for 5 min, 10% for 10 min, 30% for 25 min, 60% for 40 min, 100% for 50 min, 100% for 60 min, and 5% for 65 min, with a solvent flow of 0.8 mL/min. The volume of injection was 20 μL and the wavelength of the diode array was fixed at 360 nm.
Quercetin and myricetin have been identified according to their chromatographic retention times and UV spectra in comparison with those of authentic standards; quercetin-3-glucoside and myricetin-3-glucoside have been identified from the disappearance of single peaks and an increase in the corresponding aglycons in the HPLC chromatogram, after treatment with β-glucosidase and 1 h incubation at 35°C (Vrhovsek et al., 2004) of the extracts prepared as above, deprived of solvent and dissolved in citrate-phosphate pH 5.0 buffer; quercetin-3-glucuronide and myricetin-3-gluc-uronide have been identified from their UV spectra (similar to the ones of their glucoside derivatives) and the HPLC profile reported for the grape flavonols by Castillo-Muñoz et al. (2007).
Wines empíoyed for quercetin soíubiíity tests The selection of the wine typologies employed for our experiment was done based on different chemical compositions that were needed to test our hypothesis and based on susceptibility to quercetin precipitation. Specifically, three types of wines in which the precipitation of quercetin has never been identified, namely Barbera (from the 2018 vintage), which is rich in anthocyanins and poor in flavanols (Cagnasso et al., 2008), red Cirò (Gaglioppo-based wine from the 2014 vintage), which is poor in anthocyanins and rich in flavanols (Bosso et al., 2019), and white Cirò (Greco bianco-based wine from the 2018 vintage), in which no anthocyanins are present, and then one typology of wine in which quercetin precipitation has been identified, namely Sangiovese (from the 2014 vintage).
Different levels of anthocyanins were chosen to test the influence of these substances on the solubility of quercetin in wine, and different ages and levels of flavanols were chosen to have variability in the polymerisation level of the colorant matter.
We chose a specific Sangiovese that has previously undergone precipitation of quercetin and in which we determined the profile of the flavonols both before precipitation (October 2017) and at the time of the event itself (March 2020).
Determination of quercetin solubility in pH 3.20 buffer solution and in wines
Procedure: 1 mL of 3 g/L quercetin solution in methanol was placed in 100 mL volumetric flasks and brought to 100 mL with pH 3.20 buffer solution or with red or white wines. After stirring, the samples (three for any trial) were stored at 18°C for three days in 100 mL dark glass bottles. Other samples of the same wines and buffer solution with 30 mg/L of quercetin added were stored at 0°C for three days. Thereafter, all the samples were centrifuged for 15 min at 4 000 rpm and the liquid phases were recovered and analysed following the methods reported in this section. The controls were the wines and buffer solution to which quercetin was not added; they were stored under the same conditions as the samples to which quercetin was added. The precipitates at 18°C and
0°C were solubilised in 10 mL of methanol and, when necessary, diluted with methanol : water at 1 : 1. After recording the absorption spectrum of the methanolic solutions of precipitates from 230 to 400 nm, A371 was determined by spectrophotometry. In the liquid phases (buffer solution and wines deprived of the precipitates), A371 was determined and converted to A371 10 . In the control and the wines deprived of the precipitates, A420, A520 and total anthocyanins, total fla-vonoids and dAl + dTA and dTAT (Glories, 1984) were also determined.
Determination of total anthocyanins, total flavonoids, A420/ A520, A420 + A520, dTAT% and d'TAT%
Total anthocyanins and total flavonoids were determined by spectrophotometry after wine dilution with ethanol:H2O:conc.HCl 70:30:1 (ethanol-HCl) (Corona et al., 2015) in which the λmax of trisubstituted monomeric antho- cyanins was 540 nm. Furthermore, small flavanol-anthocy-anin polymers (Salas et al., 2004; Fulcrand et al., 2006) and flavanol-ethylidene-anthocyanin pigments (Atanasova et al., 2002) were included in the total anthocyanins. According to this method, the A540 of the wine diluted in ethanol-HCl is expressed in mg/L malvidin-3-glucoside equivalents.
A420 and A520 were determined directly in the wine and expressed as A420 10 and A520 10 . From these values it was 420, 10mm 520, 10mm possible to calculate A420/A520 and (A420 10 + A520 10 ). 420 520 420, 10mm 520, 10mm dTAT% and d'TAT% were determined respectively according to Glories (1984) and modified as reported by Corona et al. (2015). dTAT% and d'TAT% are the percentage fractions of A520 due to the non-bisulphite-bleachable pigments in wine and in wine diluted with a solution of HCl respectively.
Determination of dTAT% - In brief: 10 mL of wine and 2 mL 30% Na2S2O5 in H2O were placed in a 25 mL test tube. After stirring and 15 min of standing, the A520 of the sample was determined and expressed as A1 520 10mm.
A520 10mm = dAl + dTA + dTAT (A520 of the pigments in co- loured form at wine pH, determined in non-diluted wine) A1 520 10mm x 1.2 = dTAT (A520 of non-bisulphite-bleachable pigments in coloured form at wine pH)
dTA (A520 of bisulphite-bleachable pigments in coloured form at wine pH) dTAT% = [(A1, 520, 10mm X 1.2)/A520, 10mm] X 100
dAl = A520 of free and co-pigmented monomeric anthocya-nins in coloured form at wine pH

dTA = A520 of free and co-pigmented polymeric flavanol-anthocyanins in coloured form at wine pH Dilution factor = 1.2
Determination of d'TAT% - In brief: 1 mL wine, 1 mL 96 ethanol:HCl conc. 99.9:0.1 v:v and 20 mL conc. HCl:H2O 2:98 v:v were placed in a 50 mL flask. 10 ml of wine diluted in this manner were placed in two 25 mL test tubes, and 4 mL H2O and 4 mL 15% Na2S2O5 were added to each of the two respectively. After 15 min standing, the A'520 of the two samples was determined and these values were expressed as A'1 10mm A2, 10mm respectively.
A'1, 10mm0 χ 30.8 = d'Al + d'TA + d'TAT (A'520 of total wine pigments in coloured form in wine acidified with HCl)
A'2 10mm χ 30.8 = d'TAT (A'520 of non-bisulphite-bleachable
pigments in coloured form in wine acidified with HCl)
(A1, 10mm - A'2, 10mm) X 30.8 = d'Al + d'TA (A520 °f bisulphite-bleachable pigments in coloured form in wine acidified with HCl)

Dilution factor = 30.8
All the spectrophotometric determinations were made using an Agilent 8453 UV-Vis (Agilent Technologies, Santa Clara, CA, USA) spectrophotometer.
Statistical analysis
Statistical analyses were performed using the statistical software package SPSS (version 13.0, SPSS Inc., Chicago, IL, USA). The data analyses of the wines were processed using analysis of variance (ANOVA). To establish statistical differences by one-way analysis of variance (ANOVA), the Tukey b-test for p < 0.05 was used.
Linear regressions and Student's t test were performed with R version 4.0.3.
RESULTS AND DISCUSSION
Composition of Barbera 2018, Cirò 2014 and Sangiovese 2014 red wines
The data reported in Table 1 show that the red wines used in this study for the evaluation of quercetin solubility differed in terms of a few technological parameters (mainly ethanol % v:v and titratable acidity). The total flavonoid contents (monomeric and polymeric anthocyanins, flavanols) (Corona et al., 2015) of Barbera 2018 and Sangiovese 2014 were less than that of red Cirò 2014 (Table 2). This result was expected for Barbera, but not for Sangiovese, whose grapes usually are poor (Cagnasso et al., 2008) and rich (Benucci et al., 2018) in flavanols respectively. Different from total flavonoids, the total anthocyanin content of Barbera was much higher than that of red Cirò (made from Gaglioppo grapes rich in flavanols) and Sangiovese. This result was expected, as Barbera grapes usually are richer in anthocyanins than Gaglioppo and Sangiovese (Mattivi et al., 2006). Moreover, trisubstituted anthocyanins are found in Barbera and Sangiovese grapes, while those in Gaglioppo are disubstituted (Mattivi et al., 2006). In contrast to Barbera, the percentage of disubstituted anthocyanins in Sangiovese grapes could be high (Mattivi et al., 2006) and can prevail over trisubstituted ones, in particular because of climatic conditions (Rinaldi et al., 2021). Trisubstituted anthocyanins dominate in wines made with grapes from the three cultivars in question (Squadrito et al., 2010).
The fact that λmax in the VIS region of Barbera diluted with ethanol:HCl (see Materials and Methods) was 540 nm and the λmax max of red Cirò and Sangiovese diluted with ethanol:HCl was about 520 nm also suggests that monomeric anthocyanins, small anthocyanin-flavanol polymers, their co-pigmented forms and probably flavanol-ethylidene-anthocyanins prevail among the pigments of Barbera, while other classes of pigments were found in red Cirò and Sangiovese. Some of the latter may derive from anthocyanins (e.g. pyranoanthocyanins, anthocyanin-flavanol pigments, flavanol-anthocyanin pigments (De Freitas & Mateus, 2011; He et al., 2012a, 2012b), others would be related to flavanols (xanthylium ions and brown polymers (Es Safi et al., 2000)), and most of them would not be bisulphite-bleachable. Among the non-bleachable pigments there are also flavanol-ethylidene-anthocyanins (Escribano-Bailón et al., 2001). On the other hand, the monomeric anthocyanins, the small flavanol-anthocyanin polymers and their co-pigmented forms are bleachable (Escribano-Bailón et al., 2001).
The dTAT% (see Materials and Methods) of red Cirò 2014 and Sangiovese 2014 (49% and 47% respectively) shows that non-bleachable pigments are responsible for a large part of their A520. The contribution of non-bleachable pigments to A520 of Barbera 2018 was much lower (dTAT% = 23%). The same conclusion can be drawn from the d'TAT% values of the wines diluted with HCl (Table 2). Barbera was richer than red Cirò and Sangiovese and red Cirò richer than Sangiovese in the bleachable and non-bleachable pigments contributing to the wines' A520, as shown by the absolute values of dAl + dTA (4.870, 1.078 and 0.538 respectively) and dTAT (1.460, 0.997 and 0.477 respectively) (Table 2). Barbera therefore had young wine characteristics, while red Cirò and Sangiovese had characteristics of wines with a middle chemical age that had not achieved phenolic stability (the state in which most pigments are not bleachable, and in which the values of dTAT% and d'TAT% tend to coincide) (Di Stefano, personal communication, 2021). The dTAT% and d'TAT% values, and the A420/A520 ratio (1.20 in red Cirò, 1.16 in Sangiovese), suggest that a significant part of the A520 of the wines from 2014 was due to pyranoanthocyanins (λmax = 500 nm) and/or to other non-bleachable pigments derived from anthocyanins and/or flavanols. Otherwise, A420/A520 = 0.58 in Barbera suggests that monomeric anthocyanins and other bleachable pigments contribution to A520 wine value was prevalent. Nevertheless, the high absolute contribution of non-bleachable pigments and the λmax = 540 nm of the wine diluted with ethanol:HCl suggest that the non-bleachable pigments of Barbera could be flavanol- ethylidene-anthocyanins. Although differences were evident between the red Cirò/Sangiovese and Barbera wines, the differences between red Cirò and Sangiovese only concern the values of A420, A520, A371, A420 + A520, dTAT, dAl + dTA, and the total flavonoid contents. All other colour parameters are practically the same: pH, dTAT%, d'TAT% and A420/A520.
Solubility of quercetin in pH 3.20 hydroalcoholic buffer solution and in Barbera 2018, red Cirò 2014, Sangiovese 2014 and white Cirò 2018 wines
To determine quercetin solubility in hydroalcoholic buffer solution in Barbera 2018, red Cirò 2014, Sangiovese 2014 and white Cirò 2018, 30 mg/L of quercetin was added to them and, after the precipitation of the over-soluble concentration, we established the value of interest. We chose this amount of quercetin because it is much higher than that found by Gambuti et al. (2020) and the literature reported therein. The 30 mg/L quercetin solution in methanol:H2O 1:1 v:v added to the hydroalcoholic solution and the wines showed λmax = 371 nm and A371, 10mm = 2.415 a.u.
Three days after the spike of quercetin, all the samples showed a precipitate. Subsequently to the removal of these crystals, we analysed the model solution and wines, and these results are reported in Table 3.
Starting from the A371, 10mm measured in the hydroalcoholic model solution, a quercetin solubility of 1.64 mg/L at 18°C and 1.16 mg/L at 0°C (Table 3) was calculated. The quercetin solubility found in this study for hydroalcoholic solutions seems to be in accord with that reported by Gambuti et al. (2020) for a similar solution to which 30 mg/L of quercetin had been added before being stored at 20°C for 15 days. Also, the solubilised quercetin in Barbera, red Cirò, Sangiovese and white Cirò was calculated using the values of ΔA371 10mm (Tables 2 and 3). Barbera treated solubilised 24.3 mg/L of quercetin (Table 3). If we add this value (24.3 mg/L) to the amount of quercetin already present in the control wine (3.5 mg/L, Table 4), the solubility of quercetin in this specific Barbera at 18°C becomes 27.8 mg/L. In the same wine, to which 30 mg/L quercetin has been added before storage at 0°C for three days, the solubilised quercetin was 19.4 mg/L. This decrease in quercetin content is the result of the relationship between the solubility of a solid and temperature.
The red Cirò control contained 3.0 mg/L of quercetin (Table 4), while our experiments at 18°C showed a solubilisation of 13.0 mg/L and the 0°C trial showed 9.6 mg/L of solubilised quercetin (Table 3). By adding these values to the original content of quercetin in red Cirò, the solubility under these conditions become 16 mg/L at 18°C and 12.6 at 0°C. Regarding the white Cirò, we did not find any presence of quercetin before the addition of 30 mg/L of the same flavonol, which means that the solubilised quercetin could be regarded as equal to the solubility. In our study, we observed a solubilisation of quercetin of 7.30 mg/L at 18°C and 5.51 mg/L at 0°C. Regarding the solubilisation in Sangiovese wine, we found 3.5 mg/L of quercetin for the 18°C trial and 2.4 mg/L quercetin for the 0°C trial (Table 3). If the contents of quercetin solubilised are added to the Sangiovese control concentration (5.3 mg/L, March 2020), the solubility of quercetin in this specific wine determined at 18°C is 8.8 mg/L and 7.7 mg/L at 0°C.
This result shows that the solubility of quercetin in Sangiovese wine in March 2020 (8.8 mg/L) was higher than the content found in the wine (5.3 mg/L). The amount of quercetin found in the wine in October 2017 (11.3 mg/L, Table 4) probably was also lower than its solubility at that date, which suggests that the quercetin content of the 2014 Sangiovese was even higher before the start of the ageing process. The decrease in the solubility of quercetin over time has recently been reported by Gambuti et al. (2020). As reported above, a quercetin precipitate in the bottle of Sangiovese wine selected for this study has been observed since October 2017 (amount not determined). In the bottle sampled on March 2020, 4.8 mg of precipitated quercetin was found - lower than that estimated from the decrease of quercetin in solution in the period between the two analyses (6 mg/L), plus the quercetin theoretically produced by the hydrolysis of its main derivatives (2.2 mg/L) and the quercetin previously precipitated (not determined). This could be due to the variability between bottles (not determined), or to the fact that part of the quercetin released by the hypothetical soluble complexes in which it was involved had undergone degradation reactions, due to quercetin's considerable tendency to be oxidised (Danilewicz, 2003).
Phenolic compounds are the major candidates that form with quercetin soluble complexes from which they could be released, as their structure undergoes profound changes during the maturation and storage of wine. The influence of other compounds whose changes could be significant during the ageing and storage of wine (e.g. volatile compounds) appears unlikely, as no interaction has been reported between them and quercetin. The same can be said for the other wine compounds, as they remain virtually unchanged over time and cannot influence the evolution of quercetin solubility. Given that the phenolic composition of Sangiovese 2014 was not determined in October 2017, but only in March 2020, it is not possible to directly assess the influence of the evolution of these compounds on quercetin solubility. However, some information can be obtained from the comparison of phenolic compositions determined in Sangiovese 2014, Cirò 2014 and Barbera 2018 in March 2020, in which very different amounts of the added quercetin were solubilised. The composition of the three red wines in question (Table 2) suggests that the quercetin amount solubilised in them probably did not depend on the flavanol content. In fact, although the total flavonoids in red Cirò and Sangiovese were higher than and similar to those in Barbera respectively (Table 2), the solubility of quercetin in them was lower. The presence of a higher anthocyanin content in Barbera compared to red Cirò and Sangiovese (Table 2) also suggests that the higher solubility of quercetin in Barbera could be due to its high content in the compounds that form with quercetin-soluble complexes (co-pigmentation complexes) (Baranac et al., 1997). At the same time, because red Cirò and Sangiovese are poor in monomer anthocyanins, their quercetin over-solubility compared to the hydroalcoholic buffer solution could be due to polymeric pigments and/or other unidentified substances.
The fact that Barbera was much richer than red Cirò and Sangiovese in anthocyanins and other bisulphite-bleachable pigments, as is evident from dAl + dTA (Table 2), suggests that it was the absolute content of these compounds that affected the amount of quercetin solubilised. On the other hand, there is a positive correlation between dAl + dTA and quercetin solubility.
It can be seen from Fig. 1a and 1b and Table 5 and Table 6 that there is a significant positive linear relationship between the concentration of bleachable pigments (monomeric anthocyanins and small polymers of the colouring matter) and quercetin solubility in wines.
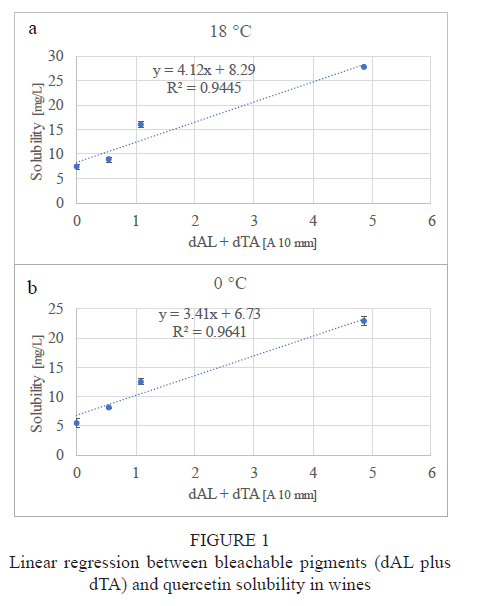
At each temperature, both the intercepts and the slopes were significantly different from zero (Table 5 and Table 6), which suggests (in the case of the intercept previously explained) that there is dependence between the solubility of quercetin and bleachable pigments, while in the case of the intercept, as it was already it was clear earlier that a certain solubility of quercetin occurs at a zero value of bleachable pigments.
If the bleachable pigments are the only compounds capable of increasing the solubility of quercetin in wines, we would expect that the intercept values obtained in our linear models would be similar to the solubility values determined in the model solutions, as happens in the case of white Cirò, as the model solution, as in the case of the wine just mentioned, is devoid of bleachable pigments both in the monomeric and polymeric form. However, observing the solubility values in Table 7, the solubility of quercetin in the model solution is lower than that in white Cirò, and occurs consistently for both temperatures at which the determinations were done.
These differences are also strongly supported statistically by Student's t test (p (t) < 0.001). This indicates that there are other substances capable of increasing the solubility of quercetin in wines besides bleachable pigments.
The exponential trend of the line that fits with the experimental data (Table 5 and Table 6) shows that the higher the content of bleachable pigments (proportional to dAl + dTA), the higher the solubility of quercetin. An influence from the non-bleachable pigments appears unlikely, as their absolute amount in Barbera (the wine richer wine in solubilised quercetin) was not much higher than that in red Cirò (dTAT10mm 1.46 a.u. and 0.99 a.u. respectively), while the absolute content of bleachable pigments was much higher (dAl + dTA 4.87 a.u. and 1.09 a.u. respectively). Sangiovese, in which the solubility of quercetin was lower, represents a separate case, as it was poorer in both total flavonoids and pigments than Barbera and red Cirò (Table 2). However, the content of bleachable pigments found in red Cirò and Sangiovese (dAl + dTA 1.09 a.u. and 0.54 a.u. respectively) could be due their quercetin over-solubility compared to the hydroalcoholic solution, which did not contain pigments. The difference between bleachable pigment contents (dAl + dTA 1.09 a.u. and 0.54 a.u. respectively) could also explain the difference in quercetin solubilised in red Cirò and Sangiovese. Indeed, the quercetin decrease in Sangiovese from 2017 to 2020 (Table 4) could be due to the decrease in bleachable pigments and the increase in non-bleachable ones during this time.
The decrease in bleachable pigments and the increase in non-bleachable ones in red wine is supported by the literature. The absence of quercetin precipitate in red Cirò and its presence in Sangiovese wines could be due to their initial content of quercetin and quercetin glycosides, which is generally much higher in Sangiovese (Gambuti et al., 2020) than in Cirò (see Mattivi et al. (2006) for the flavonol content of grapes). On the other hand, the above results prove that at least some of the quercetin that can exist in solution in a wine depends on the wine content of bleachable pigments that form with quercetin-soluble co-pigmentation complexes. The evolution of anthocyanins and other bleachable pigments to produce non-bleachable pigments during wine ageing and storage is due the release of quercetin from soluble co-pigmentation complexes and its precipitation.
If the soluble complexes in which quercetin is involved are co-pigmentation complexes,

and/or

can be the relationships that bind quercetin (Q), flavylium (AH+) or quinonoid (A) monomers and small polymer fla-vanol-anthocyanin and co-pigmentation complexes (AHQ+, AQ) (Brouillard et al., 1991). These equations also show that, as the anthocyanins decrease (polymerisation, degradation, other reactions), the equilibrium shifts to the left, with quercetin release that can precipitate if its content exceeds its solubility in that particular wine, or degraded if it is involved in oxidation reactions. Therefore, the quercetin decrease from 11.3 mg/L to 5.3 mg/L in Sangiovese 2014 from October 2017 to March 2020 could be due to its precipitation and/or degradation after its release from the co-pigmentation complexes. The solubilisation in March 2020 of 3.5 mg/L of added quercetin in Sangiovese 2014 also suggests that, besides precipitating, part of the quercetin in solution in wine in October 2017 (11.3 mg/L) and that produced by hydrolysis of its glycosylated derivatives, undergoes degradation reactions, reaching a lower content (5.3 mg/L) than what the wine was able to solubilise (8.8 mg/L) at that date. On the other hand, the comparison between flavonol composition in
October 2017 and at the end of March 2020 (Table 4) allows the observation that the hydrolysis of flavonol glyco-sides continued over time (theoretical quercetin produced 2.2 mg/L), albeit slowly, and was not yet complete in March 2020. In accordance with Spayd et al. (2002), the hydrolysis rate of quercetin-3-glucoside could be higher than for quer-cetin-3-glucuronide.
The AA371 10mm for the white Cirò 2018 samples spiked with 30 mg/L quercetin and the white Cirò 2018 control, some of which was stored at 18°C and some at 0°C for three days, were 0.59 a.u. and 0.45 a.u. respectively (Table 3). From these values, a quercetin solubility of 7.3 mg/L at 18°C and 5.5 mg/L at 0°C was calculated (Table 3), and these results show that the amount of solubilised quercetin in white Cirò 2018, both at 18°C and at 0°C, was lower than quercetin solubility in Barbera 2018 and red Cirò 2014. This is quite similar to that of Sangiovese 2014, but higher than in the buffer solution at pH 3.20. However, one cannot exclude that substances other than polyphenols influence the solubilised quercetin. These values also suggest that quercetin solubility could reach about 7 mg/L at 18°C in wines in which there are no anthocyanins, or that most of them were transformed into non-bleachable polymeric pigments. However, as demonstrated by the 2014 Sangiovese, in aged red wines that were initially rich in quercetin yet contained bleachable pigments, and in which a higher content than 7 mg/L was expected, a smaller amount could be present as some of it involved in oxidation reactions might have been degraded.
It is interesting to note that the addition of quercetin to Barbera, red Cirò and Sangiovese induced an increase in A371, but not in A520 (at 18°C and 0°C) compared to their respective controls (Table 2), unlike the expected hyperchro-mic effect induced by co-pigmentation complexes formation between pigments and solubilised quercetin. In contrast, a small decrease was found, probably due to co-precipitation
of some class of wine pigments with the added quercetin, or to a shift of the λ max towards higher values (bathochro- mic effect, not determined). The lack of hyperchromic effect could be due to the fact that it was evident with co-pigment/ anthocyanin ratios higher than those in this work (Baranac et al., 1997). Furthermore, in order to observe a hyperchro-mic effect of the co-pigmentation complexes in which small flavanol-anthocyanin polymers are involved, a much higher co-pigment/pigment ratio is required (Salas et al., 2004).
CONCLUSIONS
The data presented in this work shows that the quercetin solubilised in three red wines (Barbera 2018, red Cirò 2014, Sangiovese 2014) reaches much higher concentrations than in a pH 3.20 buffer solution containing 12% v:v ethanol, and that the over-solubility of quercetin depends on the wines' contents of monomeric and bisulphite-bleachable polymeric pigments. Quercetin over-solubility was also observed in white Cirò 2018, a white wine examined in this work. In this wine, about 7 mg/L had been solubilised at 18°C, which probably also represents the limit of quercetin that can be found in solution in red wines in which most of the monomeric anthocyanins and their bleachable polymeric derivatives have been transformed into non-bleachable pigments. The decrease in the quercetin content found in wines over time could be due to the evolution of anthocyanins and bleachable polymeric pigments, and to oxidative degradation reactions in which it is involved (probably the case in the Sangiovese 2014).
To explain the quercetin over-solubility in red wines rich in monomeric anthocyanins and their bleachable polymeric pigments (as in the case of Barbera 2018), we propose that it is the formation of soluble co-pigmentation complexes between quercetin and anthocyanins (as of 2014) that could prevent quercetin precipitation. As monomeric anthocyanins and bleachable polymeric pigments bound to quercetin in co-pigmentation complexes evolve into other molecular structures (e.g. non-bleachable pigments), part of the quercetin released by the dissociation of complexes could precipitate if it exceeds its solubility level in wine (which depends on the wine pigment composition), part could be degraded, probably through oxidation reactions, and part could remain in solution if its solubility level in wines is not reached. This mechanism seems to be proven by what was observed in Sangiovese 2014, which in March 2020 was poor in monomeric anthocyanins and bleachable polymeric pigments, with a precipitate of quercetin in the bottles. The fact that the content of quercetin was 5.3 mg/L in March 2020 (in October 2017 it was 11.3 mg/L), were solubilised 3.5 mg/L of the added quercetin (30 mg/L), proves that quercetin released from the hypothetical co-pigmentation complexes in part precipitated and in part was degraded. Its content in the wine therefore was lower than its actual solubility.
The higher quantity of solubilised quercetin in white Cirò 2018 (in which anthocyanins and related pigments were absent) compared to the hydroalcoholic buffer at pH 3.20 suggests that non-phenolic substances probably also prevent the formation of quercetin crystals. The limit of this work lies in the fact that their nature remains unidentified. On the other hand, the refrigeration of wines in which there are no pigments, but only hypothetical substances that would inhibit the growth of its crystals (e.g. white wines), shows that quercetin precipitation may also be due to quercetin solubility decreasing with a decrease in temperature.
LITERATURE CITED
Atanasova, V., Fulcrand, H., Le Guernevé, C., Cheynier, V. & Moutounet M., 2002. Structure of a new dimeric acetaldehyde malvidin 3-glucoside condensation product. Tetrahedron Lett. 43(35), 6151-6153. [ Links ]
Baranac, J.M., Petranovic, N.A. & Dimitric-Markovic, J.M., 1997. Spectro-photometric study of anthocyan copigmentation reactions. 2. Malvin and the nonglycosidized flavone quercetin. J. Agric. Food Chem. 45(5), 1694-1697. [ Links ]
Benucci, I., Cerreti, M., Liburdi, K., Nardi, T., Vagnoli, P., Ortiz-Julien, A. & Esti M., 2018. Pre-fermentative cold maceration in presence of non-Sac-charomyces strains: Evolution of chromatic characteristics of Sangiovese red wine elaborated by sequential inoculation. Food Res. Int. 107, 257-266. [ Links ]
Bosso, A., Guaita, M., Panero, L., Motta, S., Petrozziello, M., Tsolakis, C. & Sansone, L., 2019. Chemical and sensory characteristics of Gaglioppo wines obtained from grapes grown under two different water regimes. BIO Web of Conferences, Proc. 42nd World Congress Vine Wine 02008, 15. [ Links ]
Boulton, R., 2001. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 52, 67-87. [ Links ]
Brouillard, R., Wigand, M.-C., Dangles, O. & Cheminat, A., 1991. pH and solvent effect on the copigmentation reaction of malvin with polyphenols, purine and pyrimidine derivatives. J. Chem. Soc., Perkin Trans. 2 8(1991), 1235-1241. [ Links ]
Cagnasso, E., Rolle, L., Caudana, A. & Gerbi, V., 2008. Relationship between grape phenolic maturity and red wine phenolic composition. Ital. J. Food Sci. 3(20), 365-380. [ Links ]
Castellarin, S.D., Matthews, M.A., Di Gaspero, G. & Gambetta, G.A., 2007. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 227, 101-112. [ Links ]
Castillo-Muñoz, N., Gómez-Alonso, S., Garciäa-Romero, E. & Hermosin-Gutiérrez, I., 2007. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 55(3), 992-1002. [ Links ]
Cheynier, V. & Rigaud, J., 1986. HPLC separation and characterization of flavonols in the skins of Vitis vinifera var. Cinsault. Am. J. Enol. Vitic. 37(4), 248-252. [ Links ]
Corona, O., Squadrito, M., Vento, G., Tirelli, A. & Di Stefano, R., 2015. Over-evaluation of total flavonoids in grape skin extracts containing sulphur dioxide. Food Chem. 172, 537-542. [ Links ]
Cortell, J.M. & Kennedy, J.A., 2006. Effect of shading on the accumulation of flavonoid compounds in (Vitis vinifera L.) Pinot noir and extraction in a model system. J. Agric. Food Chem. 54(22), 8510-8520. [ Links ]
Danilewicz, J.C., 2003. Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: Central role of iron and copper. Am. J. Enol. Vitic. 54(2), 73-85. [ Links ]
De Freitas, V. & Mateus, N., 2011. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 401, 1463-1473. [ Links ]
Downey, M.O., Harvey, J.S. & Robinson, S.P., 2003. Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.). Aust. J. Grape Wine Res. 9(2), 110-121. [ Links ]
Downey, M.O., Harvey, J.S. & Robinson S.P., 2004. The effect of bunch shading on berry development and flavonoid accumulation on Shiraz grapes. Aus. J. Grape Wine Res. 10(1), 55-73. [ Links ]
Escribano-Bailón, T., Álvarez-García, M., Rivas-Gonzalo, J.C., Heredia, F.J. & Santos-Buelga, C., 2001. Color and stability of pigments derived from the acetaldehyde-mediated condensation between malvidin-3-O-glu-coside and (+)-catechin. J. Agric. Food Chem. 49(3), 1213-1217. [ Links ]
Es-Safi, N.-E., Le Guernevé, C., Fulcrand, H., Cheynier, V. & Moutounet, M., 2000. Xanthylium salts formation involved in wine colour changes. Int. J. Food Sci. Technol. 35(1), 63-74. [ Links ]
Fulcrand, H., Dueñas, M., Salas, E. & Cheynier, V., 2006. Phenolic reactions during winemaking and aging. Am. J. Enol. Vitic. 57(3), 289-297. [ Links ]
Gambuti, A., Picariello, L., Rinaldi, A., Forino, M., Blaiotta, G., Moine, V. & Moio, L., 2020. New insights into the formation of precipitates of quercetin in Sangiovese wines. J. Food Sci. Tech. 57(7), 2602-2611. [ Links ]
Glories, Y., 1984. La couleur des vins rouges. 2e parti e: mesure, origine et interpretation. J. Int. Sci. Vigne Vin 4(18), 253-271. [ Links ]
Haselgrove, L., Botting, D., Van Heeswijck, R., H0J, P.B., Dry, P.R., Ford, C. & Land, P.G.I., 2000. Canopy microclimate and berry composition: The effect of bunch exposure on the composition of Vitis vinifera L cv. Shiraz grape berries. Aus. J. Grape Wine Res. 6(2), 141-149. [ Links ]
He, F., Liang, N.-N., Mu, L., Pan, Q.-H., Wang, J., Reeves, M.J. & Duan, C.-Q., 2012a. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 17(2), 1571-1601. [ Links ]
He, F., Liang, N.-N., Mu, L., Pan, Q.-H., Wang, J., Reeves, M.J. & Duan, C.-Q., 2012b. Anthocyanins and their variation in red wines II. Anthocyanin derived pigments and their color evolution. Molecules 17(2), 1483-1519. [ Links ]
Keller, M. & Hrazdina, G., 1998. Interaction of nitrogen availability during bloom and light intensity during veraison. II. Effects on anthocyanin and phenolic development during grape ripening. Am. J. Enol. Vitic. 49(3), 341-349. [ Links ]
Lanati, D., Cascio, P., Pollon, M., Corona, O. & Marchi, D., 2021. Effect of leaf removal and ripening stage on the content of quercetin glycosides in Sangiovese grapes. OENO One 55(4), 71-81. [ Links ]
Lanati, D., Marchi, D. & Cascio, P., 2014. Precipitati di quercetina nei vini. In Proc. 37th World Congress Vine Wine and 12th Gen. Ass. OIV, 06007, 5. [ Links ]
Makris, D.P., Kallithraka, S. & Kefalas, P., 2006. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 19(5), 396-404. [ Links ]
Marchi, D., Lanati, D., Cascio, P. & Mazza, G., 2019. Influenza della sfo-gliatura sulla sintesi della quercetina in Sangiovese. Ulteriori acquisizioni sui precipitati di quercetina nei vini. BIO Web of Conferences, Proc. 42nd World Congress Vine Wine 02010, 15. [ Links ]
Mattivi, F., Guzzon, R., Vrhovsek, U., Stefanini, M. & Velasco, R., 2006. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 54, 7692-7702. [ Links ]
Price, S.F., Breen, P.J., Valladao, M. & Watson, B.T., 1995. Cluster sun exposure and quercetin in Pinot noir grapes and wine. Am. J. Enol. Vitic. 4, 187-194. [ Links ]
Rinaldi, A., Picariello, L., Soares, S., Brandão, E., De Freitas, V., Moio, L. & Gambuti, A., 2021. Effect of oxidation on color parameters, tannins, and sensory characteristics of Sangiovese wines. Eur. Food Res. Technol. 247(12), 2977-2991. [ Links ]
Salas, E., Le Guernevé, C., Fulcrand, H., Poncet-Legrand, C. & Cheynier, V., 2004. Structure determination and color properties of a newly synthesized direct-linked flavanol-anthocyanin dimer. Tetrahedron Lett. 45(47), 8725-8729. [ Links ]
Somers, T.C. & Ziemelis, G., 1985. Flavonol haze in white wines. Vitis 24, 43-50. [ Links ]
Spayd, S.E., Tarara, J.M., Mee, D.L. & Ferguson, J.C., 2002. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 53(3), 171-182. [ Links ]
Squadrito, M., Corona, O., Ansaldi, G. & Di Stefano, R., 2007. Relazioni fra percorsi biosintetici degli HCTA, dei flavonoli e degli antociani nella buccia dell'uva. Riv. Vitic. Enol. 60(3), 59-70. [ Links ]
Squadrito, M., Corona, O., Ansaldi, G. & Di Stefano, R., 2010. Evolution of anthocyanin profile from grape to wine. J. Int. Sci. Vigne Vin 44(3), 167-177. [ Links ]
Vrhovsek, U., Rigo, A., Tonon D. & Mattivi F., 2004. Quantitation of poly phenols in different apple varieties. J. Agric. Food Chem. 52, 6532-6538. [ Links ]
Ziemelis, G. & Pickering, J., 1969. Precipitation of flavonols in a dry red table wine. Chem. Ind. 49, 1781-1782. [ Links ]
Submitted for publication: May 2022
Accepted for publication: September 2022
* Corresponding author: E-mail address: direzione@enosis.it













