Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151Print version ISSN 0038-2361
S. Afr. j. surg. vol.62 n.1 Cape Town 2024
https://doi.org/10.36303/SAJS.00161
UPPER GASTRO-INTESTINAL SURGERY
Perforated peptic ulcer – a case series and an African perspective
MT Mahlefahlo; OD Montwedi; VOL Karusseit
Department of Surgery, Faculty of Health Sciences, University of Pretoria, South Africa
ABSTRACT
BACKGROUND: Perforation of peptic ulcer (PPU) is the most morbid complication of peptic ulcer disease (PUD) with scant recent reports from sub-Saharan Africa. The aim of this study was to describe a PPU series from a single centre in South Africa and contrast the findings with other recent reports from sub-Saharan Africa
METHODS: A retrospective study of PPU at Kalafong Hospital in Pretoria was performed. The relationship of patient demographics, risk factors, ulcer pathology and severity scores to mortality were analysed. Recent similar reports from sub-Saharan Africa were reviewed and the findings compared to the current study and findings from high income countries (HIC
RESULTS: The study comprised 121 patients. The majority were black men with an average age of 46.6 years, with few comorbidities. A large proportion of patients admitted to risk factors and most presented to hospital 48 hours after the onset of symptoms. The majority (71%) of the perforations occurred in the stomach. The patient sex, age, risk factors and the mortality at 4% were similar to other African studies, although perforations were mainly duodenal in most of the African studies. The median age of patients in the East African studies was lower by 13 years. Patients in HIC series of PPU were older, more likely to be female, have duodenal perforations and a higher mortality than in the African series
CONCLUSION: Patients were mostly smokers, presented late to hospital and had gastric perforations. The findings of low mortality and male predominance concurred with those of other sub-Saharan African reports and were the opposite of trends in HIC
Keywords: peptic ulcer, perforation, Africa
Introduction
Major changes in the epidemiology and management of peptic ulcer disease (PUD) and its complications have occurred in recent decades.1 Medical treatment is highly effective since the development of effective gastric acid suppression with proton pump inhibitors, and antimicrobial eradication regimens for H. pylori. Despite this success there is still a significant incidence of PUD complications that can lead to serious morbidity and mortality. Though less common than bleeding PUD, perforated peptic ulcer (PPU) is an emergency that requires surgery and has a higher mortality rate than bleeding.2 PPU constitutes about 10% of patients hospitalised for PUD in the United States of America (USA) and is responsible for 37% of deaths.3 PUD was historically considered to be rare in Africa.4 This is certainly no longer the case. The age standardised prevalence rate for African countries in 2019 varied from 50 to 340 per 100 000.1 The rate for South Africa was estimated to be 50-75 per 100 000. Given the gravity of visceral perforation, PPU remains an important surgical emergency.
Clinical reports are important in highlighting regional differences in the epidemiology and outcome of PPU. In high income countries (HIC) PPU is mostly a disease of the elderly, who have concomitant comorbidities.56 In low-to middle-income countries (LMIC) the age incidence is lower but is increasing, and female gender incidence is increasing.7 In sub-Saharan Africa and similar LMIC PPU occurs predominantly in males and poses several challenges that may adversely affect the outcome of patients due to late presentation, deficient hospital services and inadequate surgical and intensive care unit (ICU) facilities. Recent descriptive studies from sub-Saharan Africa are mostly from West and East Africa,8-19 with only one study each from Malawi20 and Zambia,21 and two from South Africa in 200522 and in 1989.23
The aim of this study was to describe the demographics, clinical presentation, pathology, management, and outcome of a cohort of patients with PPU from a single centre in South Africa and to compare the findings with those of other recent PPU studies from sub-Saharan Africa.
Methods
A retrospective cross-sectional study of adult patients (> 18 years) treated for PPU in the period 2012-2016 at Kalafong Hospital was undertaken. This facility is an urban tertiary hospital, which serves a mixed urban and rural population and is part of the training platform of the University of Pretoria. The catchment population of the hospital is 585 000 and is of black and white ethnicity. Data of consecutive patients identified from the Department of Surgery database were extracted from hospital records. All patients had been subjected to laparotomy because of acute abdominal pain and peritonism. Patients treated conservatively for PPU were excluded. Intraoperative perforation biopsies were performed at the discretion of the operating surgeon based on a suspicion of malignancy. Patients with gastric perforation were subjected to follow-up gastroscopy. The following data were recorded: patient demographics, history of previous PUD, risk factors of smoking or use of alcohol or anti-inflammatory drugs, time to presentation at the hospital and to surgery, and comorbidities. HIV was recorded only if known as testing was not routinely performed. In addition, patients were classified according to three prognostic risk systems: the Boey (specific for PPU), the American Society of Anaesthesiologists (ASA) grading and the Mannheim Peritonitis Index (MPI).24
Surgical findings and procedures were recorded. Outcomes which were determined were mortality, ICU admission, complications and duration of hospital stay.
The literature of the preceding 10 years (2011-2021) was searched in Medline and Google Scholar for sub-Saharan Africa publications on PPU. Articles were selected that reported on at least 50 patients and contained specific data on demographics, PPU risk factors, time to presentation, ulcer site, ulcer size and patient mortality. The data were tabulated for comparison between studies and with the current study.8-19
Statistical analysis
Data of patients in this study were entered into an Excel spreadsheet for analysis. Descriptive statistics were derived. Fisher's exact test was used to calculate the association of categorical variables with patient mortality, and the Student's t-test for comparison of means of prognostic score groups. Statistical significance was set at a p-value of 0.05.
Results
The data of 121 patients are presented. The characteristics of the patients are depicted in Table I. None of the categorical variables were statistically associated with mortality. The vast majority of patients were black men and the male to female ratio was 2.36:1. A fifth of patients had a previous diagnosis of PUD. Most patients had pain for more than two days prior to admission. A substantial proportion of patients admitted to regular use of one or more risk substances for PUD, 70% being smokers. Thirty-one patients knew their HIV status, which was positive in 11. The Boey and ASA scores did not correlate with mortality. The MPI, however, significantly predicted death in this cohort. For one patient who died, the delay to presentation and the Boey score had not been recorded.

Table II depicts operative details. All but one patient who had laparoscopy were managed by laparotomy. A substantial number of patients had purulent fluid in the abdomen. In two thirds of the patients, the surgeon reported a gastric perforation, which was less than 1 cm in size. These included corpus, antral and pre-pyloric ulcers. Perforations were repaired in all but one case with a simple omental patch with or without prior suturing. A partial gastrectomy was performed for the 3 cm ulcer perforation.
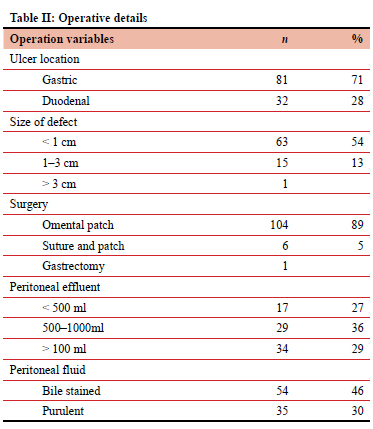
Clinical outcomes are shown in Table III. The in-hospital mortality of this group was low. Twenty-five patients were treated in ICU after surgery. Eight patients suffered acute kidney injury, of whom five died. The 116 survivors were hospitalised for a mean of 6.25 days.
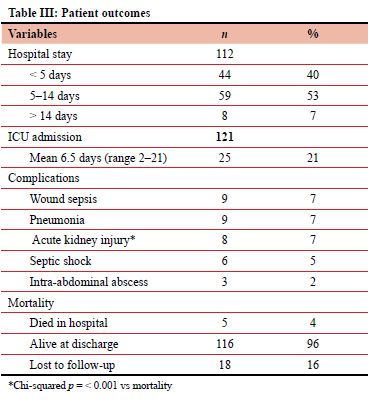
Table IV depicts data from the selected recent cross-sectional studies from sub-Saharan Africa, including the current one. These countries are all low incone countries (LIC) or LMIC. In summary, differences and similarities between various populations are: patients are relatively young, with mean ages ranging between 28 and 50 years; men on average constitute 81% of cases; smoking and the use of alcohol are highly prevalent risk factors except in the East African studies; non-steroidal anti-inflammatory drugs (NSAIDs) usage was very common; delay to presentation was common in many of the studies; in most studies, duodenal perforation was more common than gastric; and the mortality rates were relatively low, ranging between 3% and 18%.
Discussion
We present a description of PPU patients treated in a single South African institution over a five-year period. A recent review of perioperative mortality due to complicated PPU from countries in Africa reports South Africa as having the highest mortality.7 This review, however, includes historical data starting in the 1980s which cannot realistically be compared to more recent findings. The authors state that too few reports of sufficient quality emanated from South Africa. There are indeed no recent descriptive reports on PPU from South Africa. Recent sub-Saharan African publications have all been local or single centre descriptive studies from West and East Africa. Unfortunately, there are no national or regional epidemiological studies over time from Africa regarding PUD or its complications. Nevertheless demographic, pathological and outcome differences between and within HIC and LMIC can be gleaned from regional and single centre observational studies, such as the current one.
The vast majority of PPU patients in the current report were men. In general, this concurs with the sex distribution pattern in Africa as shown in Table IV where males constituted on average 81% of the series. Similarly in the African review by Peiffer et al., men constituted 79% of PPU cases and with incidences varying from 6 to 13 times higher than in females.7,25 This is in contrast to the male to female distribution in HIC, which is closer to parity.25-28 The most plausible reason for this is that smoking is known to be more prevalent amongst men in LMIC.29
While there was a wide age range of patients in the present study, the mean age of patients of 46.6 years concurs with the studies from West Africa. PPU patients from East Africa are even younger with mean ages of patients in Ethiopia, Tanzania and Somalia ranging from the late twenties to middle thirties.12-19 (Table IV). These mean ages in Africa are in stark contrast to those in HICs, which have been reported as 62 years in the USA,26 57 years in England 27 and 69 years in Norway,4 and have been increasing in recent decades.430 This is thought to be due to the increasing use of NSAIDs in the elderly.4 Age trends of PPU over recent decades in Africa cannot be accurately discerned because of the lack of epidemiological studies on the continent.
The known risk factors of PUD were common in the current study. This is probably related to the predominantly urban and peri-urban composition of the catchment population. Almost half of the patients admitted to using NSAIDs or alcohol on a regular basis. This is similar to other African studies. In East African studies NSAIDs use was uncommon. The majority of patients in the current study admitted to smoking, which was common in all African studies. Smoking and NSAID use are particularly associated with PPU.31,32 While smoking is declining globally, this is mainly in HIC. There has been little decline in LIC and LMIC where 80% of tobacco consumption occurs.33 In several of the East African PPU studies, usage of Khat, a chewable stimulant leaf, has been reported as a risk factor.12-14 There are, however, no controlled studies demonstrating its aetiological role in PUD.
There is a wide range of anatomical site distribution of PPU (Table IV). The majority (71%) of the ulcers in the present study were located in the stomach, while in most of the African studies, duodenal perforation was more common than gastric which is corroborated in a recent review.25 Gastric ulcer perforations ranged from as few as 2% in an Ethiopian study12 to as many as 94% in a Ugandan study.19 While unexpected, the large proportion of gastric perforation in some of the studies should probably be accepted as broadly correct, with four of the 13 studies as well as the Zambian study21 recording predominantly gastric ulcers. One explanation may lie in the difficulty at operation of determining the exact anatomical location of a juxtapyloric perforation because of inflammation due to the ulcer and the reaction to the perforation. It may be speculated that the anatomical distribution of PUD in Africa is changing, as it has over recent decades in developed countries, in which the incidence of duodenal ulcer is increasing and gastric ulcer decreasing.5,30
The time elapsed before presentation at the hospital was more than 48 hours in 59% of patients in the current study. Delay to presentation was common in many of the studies (Table IV). Delay would be expected in LMIC because of poorer infrastructure and medical care. This is in contrast to delay in developed countries. In the quoted Norwegian study, 85% of patients presented in under 24hours.5 Delayed presentation is widely recognised to be associated with increased mortality,34 but this effect seems to be possibly counterbalanced by other factors in patients in Africa, who are younger and probably have fewer comorbidities.
Five patients (4%) died in hospital in this study. The mortality rates are relatively low, ranging between 3% and 18% (Table IV). The reasons for this are not discernible in these studies. The mortality rate tends to be higher in HIC, despite better social circumstances and medical care and is probably because of the profile of PPU patients in developed countries who are in general older and have more comorbidities.1,5,6 In the current cohort, apart from hypertension in 26 patients, comorbidities were uncommon.
This study has several limitations. Because of its retrospective nature some data are missing. Follow-up was also not complete. These factors may have influenced the incidence of morbidity and mortality. While statistical correlation of mortality with some patient characteristics is presented in this study, this cannot be accepted as robust because of the small number of deaths in several independent variable categories.
Conclusion
The PPU patients in this cohort were predominantly young males with few comorbidities. The majority were smokers and they presented late to hospital. Findings in general were similar to those of other African studies with the exception of ulcer site, which was variable and may be methodological. The mortality was low, comparable to that of other African studies. Because perforation of a peptic ulcer is probably more related to personal habits than to H. pylori infection, it may be that increasing NSAIDs use and smoking play a large part in the emergence of PPU in Africa. Preventive measures should be aimed at these risk factors through education of both patients and prescribing doctors.
Conflict of interest
The authors declare no conflict of interest.
Funding source
The costs of this study were borne by internal departmental funds only.
Ethical approval
Consent to conduct the study was granted by the Research Ethics Committee of the Faculty of Health Sciences of the University of Pretoria (Reference no. 73/2018).
ORCID
MT Mahlefahlo https://orcid.org/0000-0003-0795-2234
OD Montwedi https://orcid.org/0000-0002-2923-7920
VOL Karusseit https://orcid.org/0000-0003-0377-3941
REFERENCES
1. Xie X, Ren K, Zhou Z, Dang C, Zhang H. The global, regional and national burden of peptic ulcer disease from 1990 to 2019: A population-based study. BMC Gastroenterol. 2022;22(1):58. https://doi.org/10.1186/s12876-022-02130-2. [ Links ]
2. Lau JY, Sung J, Hill C, et al. Systematic review of the epidemiology of complicated peptic ulcer disease: Incidence, recurrence, risk factors and mortality. Digestion. 2011;84(2):102-13. https://doi.org/10.1159/000323958. [ Links ]
3. Wang YR, Richter JE, Dempsey DT. Trends and outcomes of hospitalisations for peptic ulcer disease in the United States, 1993 to 2006. Ann Surg. 2010;251(1):51-8. https://doi.org/10.1097/SLA.0b013e3181b975b8. [ Links ]
4. Balint GA. Selected gastrointestinal pathologies in tropical sub-Saharan Africa. Bull World Health Organ. 1998;76(2):207-12. [ Links ]
5. Dadfar A, Edna TH. Epidemiology of perforating peptic ulcer: A population-based retrospective study over 40 years. World J Gastroenterol. 2020;26(35):5302-13. https://doi.org/10.3748/wjg.v26.i35.5302. [ Links ]
6. Thorsen K, Sareide JA, Kvalay JT. Epidemiology ofperforated peptic ulcer: Age- and gender-adjusted analysis of incidence and mortality. World J Gastroenterol. 2013;19(3):347-54. https://doi.org/10.3748/wjg.v19.i3.347. [ Links ]
7. Peiffer S, Pelton M, Keeney L, et al. Risk factors of perioperative mortality from complicated peptic ulcer disease in Africa: Systematic review and meta-analysis. BMJ Open Gastroenterol. 2020;7(1):e000350. https://doi.org/10.1136/bmjgast-2019-000350. [ Links ]
8. Obonna GC, Obonna MC. Peptic ulcer perforation: Experience in the riverine south-western Nigeria. Tropical Journal of Medicine and Medical Sciences. 2020;1(1):10-16. https://doi.org/10.46912/wjmbs.11. [ Links ]
9. Gbenga OJ, Ayokunle DS, Ganiyu A, et al. Pattern of presentation, management and early outcome in patients with perforated peptic ulcer disease in a semi-urban tertiary hospital. Ethiop J Health Sci. 2021;31(5):975-84. [ Links ]
10. Dongo AE, Uhunmwagho O, Kesieme EB, Eluehike SU, Alufohai EF. A five-year review of perforated peptic ulcer disease in Irrua, Nigeria. Int Sch Res Notices. 2017:8375398. https://doi.org/10.1155/2017/8375398. [ Links ]
11. Ugochukwu AI, Amu OC, Nzegwu MA, Dilibe UC. Acute perforated peptic ulcer: On clinical experience in an urban tertiary hospital in south east Nigeria. Int Surg. 2013;11(3):223-7. https://doi.org/10.1016/j.ijsu.2013.01.015. [ Links ]
12. Bupicha JA, Gebresellassie HW, Alemayehu A. Pattern and outcome of perforated peptic ulcer disease patient in four teaching hospitals in Addis Ababa, Ethiopia: A prospective cohort multicentre study. BMC Surg. 2020;20(1):135. https://doi.org/10.1186/s12893-020-00796-7. [ Links ]
13. Seyoum N, Ethicha D, Assefa Z, Nega B. Risk factors that affect morbidity and mortality in patients with perforated peptic ulcer diseases in a teaching hospital. Ethiop J Health Sci. 2020;30(4):549-58. https://doi.org/10.4314/ejhs.v30i4.10. [ Links ]
14. Teshome H, Birega M, Taddese M. Perforated peptic ulcer disease in a tertiary hospital, Addis Ababa, Ethiopia: Five-year retrospective study. Ethiop J Health Sci. 2020;30(3):363-70. https://doi.org/10.4314/ejhs.v30i3.7. [ Links ]
15. Bekele A, Zemenfes, Seyoum K, et al. Patterns and seasonal variations of perforated peptic ulcer disease: Experience from Ethiopia. The Annals of African Surgery. 2017;14 (2):86-9. https://doi.org/10.4314/aas.v14i2.7. [ Links ]
16. Bejiga B, Negasa T, Abebe A. Treatment outcome of perforated peptic ulcer disease among surgically treated patients: A cross-sectional study in Adama Hospital Medical College, Adama, Ethiopia. Int J Surg Open. 2022;48:1-5. https://doi.org/10.1016/j.ijso.2022.100564 [ Links ]
17. Chalya PL, Mabula JB, Koy M, et al. Clinical profile and outcome of surgical treatment of perforated peptic ulcers in Northwestern Tanzania: A tertiary hospital experience. World J Emerg Surg. 2011;6:31. https://doi.org/10.1186/1749-7922-6-31. [ Links ]
18. Ali AM, Mohamed AN, Mohamed YG, et al. Clinical presentation and surgical management of perforated peptic ulcer in a tertiary hospital in Mogadishu, Somalia: A 5-year retrospective study. World J Emerg Surg. 2022;17(1):23. https://doi.org/10.1186/s13017-022-00428-w. [ Links ]
19. NansubugaM, Kirunda S, WesongaAS, et al. Clinico-pathology and early post-operative complications of gastro-duodenal perforations at Mulago Hospital Kampala:A prospective cohort study. East and Central Journal of Surgery. 2016;21(2):3-8 https://doi.org/10.4314/ecajs.v21i2.1. [ Links ]
20. An SJ, Davis D, Kayange L, Gallaher J, Charles A. Predictors of mortality for perforated peptic ulcer disease in Malawi. Am J Surg. 2023;225(6):1081-5. https://doi.org/10.1016/j.amjsurg.2022.11.029. [ Links ]
21. Sondashi KJ, Odimba BFK, Kelly P. A cross-sectional study on factors associated with perforated peptic ulcer disease in adults presenting to UTH, Lusaka. Med J Zambia. 2011;38(2). [ Links ]
22. Madiba TE, Nair R, Mulaudzi TV, et al. Perforated gastric ulcer: reappraisal of surgical options. S Afr J Surg. 2005;43(3):58-60. [ Links ]
23. Schein M, Decker GA. Five hundred operations for peptic ulcer disease at JG Strijdom Hospital, 1980-1987. S Afr J Surg. 1989;27(5):161-7. [ Links ]
24. Thorsen K, Sareide JA, Sareide K. Scoring systems for outcome prediction in patients with perforated peptic ulcer. Scand J Trauma Resusc Emerg Med. 2013;21:25. https://doi.org/10.1186/1757-7241-21-25. [ Links ]
25. Sareide K, Thorsen K, Harrison EM, et al. Perforated peptic ulcer. Lancet. 2015;386:1288-98. https://doi.org/10.1016/S0140-6736(15)00276-7. [ Links ]
26. Olufajo OA, Wilson A, Yehayes B, et al. Trends in the surgical management and outcomes of complicated peptic ulcer disease. Am Surg. 2020;86(7):856-64. https://doi.org/10.1177/0003134820939929. [ Links ]
27. Johnson CH, McLean RC, McCallum I, Perren D, Phillips AW. An evaluation of the epidemiology, management and outcomes for perforated peptic ulcers across the North of England over 15 years: A retrospective cohort study. Int J Surg. 2019;64:24-32. https://doi.org/10.1016/j.ijsu.2019.03.005. [ Links ]
28. Hermansson M, Ekedahl A, Ranstam J, Zilling T. Decreasing incidence of peptic ulcer complications after the introduction of the proton pump inhibitors, a study of the Swedish population from 1974-2002. BMC Gastroenterol. 2009;9:25. https://doi.org/10.1186/1471-230X-9-25. [ Links ]
29. Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311(2):183-92. https://doi.org/10.1001/jama.2013.284692. [ Links ]
30. Wysocki A, Budzynski P, Kulawik J, et al. Changes in the localisation of perforated peptic ulcer and its relation to gender and age of the patients throughout the last 45 years. World J Surg. 2011;35(4):811-6. https://doi.org/10.1007/s00268-010-0917-2. [ Links ]
31. Gisbert JP, Legido J, García-Sanz I, Pajares JM. Helicobacter pylori and perforated peptic ulcer prevalence of the infection and role of non-steroidal anti-inflammatory drugs. Dig Liver Dis. 2004;36(2):116-20. https://doi.org/10.1016/j.dld.2003.10.011. [ Links ]
32. Zelickson MS, Bronder CM, Johnson BL, et al. Helicobacter pylori is not the predominant aetiology for peptic ulcers requiring operation. Am Surg. 2011;77(8):1054-60. https://doi.org/10.1177/000313481107700827. [ Links ]
33. Dai X, Gakidou E, Lopez AD. Evolution of the global smoking epidemic over the past half century: Strengthening the evidence base for policy action. Tob Control. 2022;31(2):129-37. https://doi.org/10.1136/tobaccocontrol-2021-056535. [ Links ]
34. Buck DL, Vester-Andersen M, Maller MH; Danish Clinical Register of Emergency Surgery. Surgical delay is a critical determinant of survival in perforated peptic ulcer. Br J Surg. 2013;100(8):1045-9. https://doi.org/10.1002/bjs.9175. [ Links ]
 Correspondence:
Correspondence:
VOL Karusseit
Email: otto.karusseit@up.ac.za













