Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.115 n.5 Pretoria Jun. 2025
https://doi.org/10.7196/SAMJ.2025.v115i5.3310
CORRESPONDENCE
Not so simple: Implementing a sputum jar to enhance TB diagnostic yield
To the Editor: In attempts to reduce sputum specimen rejection rates and improve laboratory workflow and Xpert MTB/ RIF Ultra (Cepheid, USA) test accuracy, a modified sputum jar was developed by Sinapi (Sinapi, South Africa (SA)) (https://sinapibiomedical.com/wp-content/uploads/2020/09/Specimen-Collection-Cup-IFU-l.pdf). We share lessons learnt on the Sinapi product development lifecycle and not-so-easy implementation roadmap. The Sinapi concept was proposed in 2015 to address sputum specimen rejection rates. This initiated a laboratory descriptive study that reported that of those specimens rejected, the majority were due to leaking specimen containers (61%),[1] which is a biosafety issue.
The Sinapi prototype design was available in 2017, and by 2019 our laboratory studies demonstrated that the material composition and fabrication did not inhibit standard of care processing (diagnostic molecular testing and liquid culture). Clinical research study implementation commenced in 2021 (during the COVID-19 pandemic) at a tertiary hospital in Gauteng Province, SA, with several hurdles encountered, as outlined in Fig. 1.
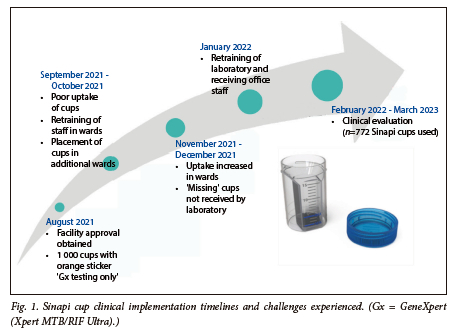
It became apparent that the sputum specimen journey post collection to laboratory referral (known as the pre-analytical phase) was not standardised across the region. Despite this, and although 59 cups could not be accounted for, the introduction of Sinapi cups resulted in a lower sputum rejection rate of 1.7% (13/772), with no rejection attributed to specimen leakage. In addition, we performed a feasibility study among healthcare and laboratory workers that generated a medium system usability score of 83 (good performance).
Overall, we learnt that although early field evaluations inform product improvements, a multi-prong approach with on-the-ground ongoing training, key opinion leader engagement and complete specimen journey data collection are integral to translational research. As Broger et al.[2] highlight, the importance of diagnostic yield (the proportion of people in whom a diagnostic test identifies tuberculosis (TB) among all people for whom testing is attempted) will be key for investigating future TB diagnostic strategies. In addition, reducing rejections will reduce loss to follow-up. We hope that our lessons learnt on simple sputum jars will be valuable to the new specimen collection strategies such as tongue swabs, and ensure that processes are developed and implemented to support the entire diagnostic value chain.
Data availability. The data supporting this study's findings are available from the corresponding author, AD, upon reasonable request and upon National Health Laboratory Service institutional approval.
Acknowledgements. The authors would like to thank Sinapi Biomedical for providing the Sinapi jars used in this study; Dr Alex Blydenstein and the ward, receiving and laboratory staff at the Chris Hani Baragwanath Hospital for their participation; Wits Diagnostic Innovation Hub TB laboratory staff; Mbuti Radebe, Dr Vidya Keshav and Silence Ndlovu.
Author contributions. AD analysed the data. LS conceptualised the investigation. All authors drafted, edited and approved the final letter.
Funding. This work was supported by the National Institutes of Health (NIH), awarded to Northwestern University, with a subaward to University of the Witwatersrand (60068868 WITS).
A David
Wits Diagnostic Innovation Hub, School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa anura.david@witsdih.ac.za
P Marokane
National Priority Programme, National Health Laboratory Service, Johannesburg, South Africa
L Singh
Wits Diagnostic Innovation Hub, School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
J E Farley
School of Nursing, Johns Hopkins University, Baltimore, USA
P da Silva
National Priority Programme, National Health Laboratory Service, Johannesburg, South Africa
W Stevens
Wits Diagnostic Innovation Hub, School of Pathology, Faculty of Health Sciences, University of the Witwatersrand; National Priority Programme, National Health Laboratory Service, Johannesburg, South Africa
L Scott
Wits Diagnostic Innovation Hub, School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
References
1. Zerbini MS, Singh S, Botha, M, Ghebrekristo Y, Opperman CJ. Specimen rejection in a high-throughput TB laboratory: A descriptive study. S Afr Med J 2023;113(10):el364. https://doi.org/10.7196/SAMJ.2023.vll3il0.1364 [ Links ]
2. Broger T, Marx FM, Theron G. et al. Diagnostic yield as an important metric for the evaluation of novel tuberculosis tests: Rationale and guidance for future research. Lancet Glob Health 2024;12(7):e1184-e1191. https://doi.org/10.1016/S2214-109X(24)00148-7 [ Links ]













