Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840XPrint version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.70 Durban 2017
https://doi.org/10.17159/0379-4350/2017/v70a21
RESEARCH ARTICLE
An initial assessment of naproxen, ibuprofen and diclofenac in Ladysmith water resources in South Africa using molecularly imprinted solid-phase extraction followed by high performance liquid chromatography-photodiode array detection
Lawrence M. MadikizelaI, *; Phumlane S. MdluliI; Luke ChimukaII
IDepartment of Chemistry, Durban University of Technology, P. O. Box 1334, Durban, 4000, South Africa
IIMolecular Sciences Institute, University of Witwatersrand, Private BagX3, Johannesburg, 2050, South Africa
ABSTRACT
In this study, the extraction of naproxen, ibuprofen and diclofenac from Ladysmith water resources was conducted by means of a multi-template molecularly imprinted polymer (MIP) as selective sorbent in solid-phase extraction. Quantification was done using high performance liquid chromatography with photo diode array detection system. Bulk polymerization of MIP was carried out at 70 °C for 24 h and characterized with differential scanning calorimetry, X-ray diffraction and zeta potential. The analytical method detection limits for naproxen, ibuprofen and diclofenac in wastewater treatment plant effluent were 0.23,1.02 and 0.30 L-1, respectively. Recoveries obtained in wastewater, river water, deionized water and drinking water treatment plant (DWTP) samples spiked with 5 L-1 of target compounds and pre-concentrated using molecularly imprinted solid-phase extraction (MISPE) were greater than 80 %. All compounds were not detected in DWTP samples, whereas in river water the concentrations were generally higher in the upstream of wastewater treatment plants compared to downstream. The maximum concentrations detected in river water for naproxen, ibuprofen and diclofenac were 2.77, 6.72 and 2.58 /ig L-1, respectively. Only diclofenac was present in wastewater at concentrations above the limit of quantification. In conclusion, the high levels of naproxen, ibuprofen and diclofenac detected in river water could be attributed to poor sanitation in Ladysmith.
Keywords: Molecularly imprinted polymer, pharmaceuticals, water resources, solid-phase extraction.
1. Introduction
Analyte extraction and pre-concentration are very crucial steps in the analysis of environmental pollutants. For instance, in environmental monitoring, both sampling and sample preparation are labour-intensive and they constitute more than 80 % of the analysis time.1 To date, several sample preparation techniques including solid-phase extraction (SPE), solid-phase microextraction, hollow fibre-based liquid phase micro-extraction and stir bar sorptive extraction have been reported for the quantitative analysis of pharmaceuticals in the environ-ment.2-5 In all these aforementioned procedures, SPE is the most used system where various traditional sorbents that include hydrophilic lipophilic balance (Oasis HLB), Oasis MCX, Strata X and C18are employed.^Most recently, the application of molec-ularly imprinted polymers (MIPs) as selective sorbents in solid-phase extraction of pharmaceuticals from aqueous samples have been reported.10-12 MIPs are gaining popularity due to their high selectivity, thermal stability, re-usability, and stability in aqueous and organic solvents.13,14
Once extracted, pharmaceuticals are usually quantified using gas chromatography (GC)15,16 and high performance liquid chromatography (HPLC).9 Derivatization is required in GC in order to improve the volatility of naproxen, ibuprofen and diclofenac.15,16 It has been reported that derivatization has a tendency to increase the analysis time and may lead to the formation of unwanted byproducts.17 Therefore, in order to avoid derivatization in GC which is one of the cumbersome steps, high performance liquid chromatography (HPLC) is often used for the quantification of naproxen, ibuprofen and diclofenac in environmental samples. Detection of pharmaceuticals using HPLC is usually carried out using photo diode array, fluorescence and mass spectrometry detectors.12,18
Naproxen, ibuprofen and diclofenac are acidic pharmaceuticals that belong to the class of non-steroidal anti-inflammatory drugs (NSAIDs), hence, they are used to treat inflammation and fever in humans.19 The consumption of such drugs is usually followed by the excretion of naproxen, ibuprofen and diclofenac with 70, 10 and 10 % as un-metabolized drugs, respectively.20 The sources of these drugs in the environment are associated with the industries that manufacture medical mixtures, disposal of expired drugs, hospital wastewater and wastes, and excretion of drugs and their metabolites by animals and humans.21
Due to these reasons, naproxen, ibuprofen and diclofenac are widely detected in water samples. In well-developed countries in Europe such as Spain, Italy, and Mexico, the evidence of environmental monitoring of pharmaceutical compounds exist.9,18,22 In this case, pharmaceuticals have been detected in wastewater influent and effluent, river water, dam water, lake water as well as in drinking water .9,18,22,23 The distribution of the same pharmaceutical drugs in the African environment is not fully known. This can be due to unavailability of sensitive instrumentation such as liquid chromatography coupled with quadrupole time of flight mass spectrometry detection, as most African research institutions are unable to afford the purchase or/and the running costs associated with this modern technology. To address this, more research is directed towards the development of sample preparation techniques that could enhance the sensitivity of less expensive laboratory instrumentation. In recent years, few papers that are based on the occurrence of naproxen, ibuprofen and diclofenac in South African wastewater and river water have been published.11,24-27 These published papers focused more on environmental monitoring of these drugs in water samples that were collected from major cities such as Durban and Johannesburg. To date, there are currently no reports on the occurrence of naproxen, ibuprofen and diclofenac in water samples collected from rural areas in South Africa. Further to this, the assessment of naproxen, ibuprofen and diclofenac in South African studies have only been conducted in wastewater and river water. Such investigations are important in rural areas due to lack of available facilities designed for the purification of water. Therefore, in most South African rural communities, untreated river water is the only water that can be used for human consumption. As well, river water is normally shared with animals such as horses, etc. In addition, rural communities do not have proper sanitation systems, hence their rivers face the risks of being heavily polluted. Therefore, it is important to monitor the pollutants in rural environment in order to prevent the health risks that could occur due to long-term consumption of polluted water.
To address these problems, this paper was aimed to provide an initial assessment of water quality in rural Ladysmith by monitoring the occurrence of naproxen, ibuprofen and diclofenac in various water resources that include the wastewater, river water and drinking water. To achieve this aim, the existing method for the synthesis of multi-template molecularly imprinted polymer, SPE application and HPLC quantification was used.11 The application of selective SPE sorbent was very important as this could enhance the detection of target compounds at low levels.
2. Experimental
2.1. Chemicals, Reagents and Apparatus
The analytical standards that were used as templates in MIP synthesis were naproxen (98 %), ibuprofen (>98 %) and diclofenac sodium salt, all purchased from Sigma-Aldrich (Steinheim, Germany). In the synthesis of MIP 2-vinylpyridine (97 %), 1,1'-azobis-(cyclohexanecarbonitrile) (98 %), ethylene glycol dimethacrylate (98 %) and toluene (99.7 %) purchased from Sigma-Aldrich (Steinheim, Germany) were used as functional monomer, radical initiator, cross linking monomer and porogenic solvent, respectively. Solvents used in template removal, solid-phase extraction and chromatographic mobile phase were methanol (>99.9 %, HPLC grade) from Sigma-Aldrich (Steinheim, Germany), acetonitrile (>99.9%,HPLC grade) from Merck (Darmstadt, Germany), glacial acetic acid (100 %) from Merck (Darmstadt, Germany) and formic acid (approx. 98 %) from Fluka (Steinheim, Germany). Deionized waterwas produced withawaterpurificationsystemfromLasec (Durban, South Africa).
2.2. Synthesis of Multi-template Molecularly Imprinted Polymer
MIP was synthesized based on the method reported by Dai el at.13and Duan el at. 23, and modified for the extraction of naproxen, ibuprofen and diclofenac in our previous work.11,19,28 In this work, 20 mg of 1,1'-azobis-(cyclohexanecarbonitrile) was dissolved in 50 mL of toluene, thereafter 1.51 mL of ethylene glycol dimethacrylate was added. Nitrogen gas was bubbled through the reaction mixture for 10 min to provide the inert atmosphere. The reaction vessel was sealed and kept in an oil bath set at 70 °C with constant stirring for 8 h. Thereafter, the following chemicals were added into the reaction mixture; naproxen (76.60 mg), ibuprofen (68.69 mg), diclofenac (106.04 mg), acetonitrile (25 mL), 2-vinylpyridine (0.25 mL), ethylene glycol dimethacrylate (3.85 mL), 1,1'-azobis-(cyclo-hexanecarbonitrile) (60 mg) and toluene (25 mL). The resulting solution was purged with nitrogen gas for 10 min and sealed. Thereafter, the polymerization reaction was refluxed at 70 °C for 16 h. The produced solid polymer was dried at 60 °C and milled. The polymer was sieved and the particles ranging from 25 to 50 μπι were collected. Non-imprinted polymer (NIP) was synthesized following similar reaction conditions with the exclusion of templates. Template molecules from the MIP were removed by washing the polymer with a mixture of acetic acid in acetonitrile (10 % (v/v)) followed by acetonitrile only. NIP was also subjected to the same washing conditions as the MIP. MIP washing solutions from each cycle were analyzed with HPLC for the presence of templates.
2.3. Characterization
Characterization of polymers was done with differential scanning calorimetry (DSC), X-ray diffraction (XRD) and zeta potential. DSC was performed using a thermal analysis instrument (model SDTQ600) from Delaware (Newcastle, USA). Both MIP and NIP were heated from 30 °C to 700 °C using a heating rate of 10 °C min-1 in DSC under nitrogen purge of 50 mL min-1. In XRD analysis, the instrument from Bruker AXS (Karlsruhe, Germany) was equipped with XRD commander for data collection and Eva software for processing. The zeta potentials of polymer particles dispersed in water were determined at 25 °C using a zeta instrument (Model: Nanosight NS 500) obtained from Malvern Instruments Limited (Worcestershire, UK).
2.4. Swelling Studies
Approximately 40 mg of each polymer was transferred into a 15 mL centrifuge tube followed by the addition of 10 mL of appropriate solvent. Solvents investigated were toluene, acetone, acetonitrile and water. The centrifuge tube was sealed and left at room temperature for 48 h, followed by centrifugation at 4000 rpm for 10 min. The excess solvent was discarded and the weight of the wet (swollen) polymer was recorded. Each experiment was done in triplicate. The swelling capacity was calculated using Equation 1:

where swelling capacity is expressed in units of % (m/m), mw is the weight of the wet MIP/NIP and md is the weight of the dry MIP/NIP.29
2.5. Sampling and Study Sites
Water samples were collected from Ladysmith water resources (Table 1 and Fig. 1) using pre-cleaned glass bottle containers. Samples were collected from the following water resources; wastewater treatment plants (WWTPs) (site numbers 3 and 6), river water (site numbers 2, 4, 5 and 7) and drinking water treatment plant (DWTP) (site number 1). Field measurements that include dissolved oxygen, conductivity, pH, total dissolved solids and salinity were recorded at the sampling sites using a portable Bante900P multi-parameter water quality meter that was purchased from Bante instruments (Shanghai, China). The collected samples were sent to the laboratory, where the suspended solids were immediately removed by filtration through a 0.45 μηι membrane filter purchased from Pall Corporation (Michigan, United States). The pH in each sample was adjusted to 2.5 with formic acid, thereafter samples were kept in the refrigerator at 4 °C until analysis.
Study sites are located in KwaZulu-Natal province which is positioned on the southeastern seaboard of the Republic of South Africa. The geographic area of the province is 94 361 km2. This province has a population of just over ten million people with a density of 110 people per km2.30 The two sampled WWTPs are known as Ladysmith and eZakheni WWTPs. Ladysmith WWTP treats sewage water from the small industries and local households located around the town. EZakheni WWTP is located in eZakheni Township and only receives wastewater from domestic sources. Water after treatment from both WWTPs is discharged into the Klip River. Sampling was done in the Klip River, upstream and downstream of the WWTPs. Within the Ladysmith region, there is a drinking water treatment facility. In this case, the water is withdrawn from the local dam for treatment prior to its release to the consumers. In this study, water prior to and post treatment in the drinking water treatment facility is referred to as raw water and effluent, respectively.
2.6. Molecularly Imprinted Solid-phase Extraction
For sample preparation, the method described previously was adopted and applied.11 This was done by packing 50 mg of MIP particles in 3 mL SPE cartridges using an acetonitrile slurry. Polypropylene frits were fitted at the bottom and top of the prepared MIP sorbent. The molecularly imprinted solid-phase extraction (MISPE) cartridge was conditioned with 2 mL of acetonitrile. Equilibration was done with 2 mL of deionized water adjusted to pH 2.5 with formic acid. With the assistance of a vacuum pump, 50 mL of the sample acidified with formic acid to pH 2.5 was loaded at 0.3 mL min-1. Thereafter, washing of the matrix interfering species was done with 2 mL of 10 % (v/v) methanol in water. The retained compounds were removed from the sorbent with 2 mL of acetic acid in acetonitrile (20 % (v/v)). For regeneration of the MIP after single application, the cartridge was flushed with acetic acid in acetonitrile (20 % (v/v)) followed by washing with 3 mL of acetonitrile.
2.7. Chromatographic Conditions
Compounds were analyzed using a HPLC system from Shimadzu Corporation (Kyoto, Japan) equipped with an online mobile phase degasser unit (Model: DGU-20A3), sample loop (20 ^L), pump (Model: LC-20AB), photo diode array detector (Model: SPD-M20A) and Shimadzu LC solutions software. 20 of the eluted extract (from MISPE) was injected into the HPLC system. The chromatographic separation was performed on a Kinetex C18 HPLC column of 150 X 4.6 mm X 2.6 μηι obtained from Phenominex (California, USA) using a mixture of acetonitrile: 0.2 % formic acid in water (60:40, v/v) as the mobile phase at a flow rate of 0.8 mL min-1. Detector wavelengths were 200 nm for monitoring ibuprofen and diclofenac while naproxen was quantified at 230 nm.
2.8. Quality Assurance and Analysis
The analytical method was verified using linearity, precision, accuracy, limit of detection (LOD) and limit of quantification (LOQ). For analysis, target compounds were identified in the chromatograms of environmental samples based on the retention times obtained from direct injection of standard solutions and those of real samples after extraction. For quality assurance, the standard solutions and prepared samples were injected in triplicate. The presence of target compounds in environmental samples were confirmed with photo diode array (PDA) spectrum for individual compounds as previously described.17 This was done by matching the spectrum of the pure compound with the one obtained during the analysis of the environmental sample. External calibration was used for quantification of naproxen, ibuprofen and diclofenac in water samples. The concentrations of five standard solutions used for the construction of calibration curves were in the range of 50 to 1000 ^gL-1 for each compound.
3. Results and Discussion
3.1. Characterization and Swelling Analysis
Characterization with DSC resulted in two endothermic peaks for naproxen which were due to the melting transition at 160 °C and thermal degradation of the drug at 270 °C, as shown in Fig. 2. These two peaks were not observed in both polymers which could be an indication of total removal and omission of templates from the MIP and NIP, respectively. It is known from its formulation that NIP did not contain any of the template molecules, therefore the similarity in the thermal behaviour of MIP and NIP could be translated as due to the removal of templates from the MIP. DSC thermograms of MIP and NIP were similar with endothermic peak at 360 °C which is associated with the thermal decomposition of both polymers.

The X-ray diffractograms (Fig. 3) indicate that the prepared polymers were amorphous in nature, due to the lack of peaks. This observation is in agreement with the study which showed the lack of crystallinity for MIP designed for abacavir (an antiviral drug) and the corresponding NIP.31 Also, similar diffracto-grams for MIP and NIP could be due to the similarities in the structural backbones of the polymers.

Zeta potentials for MIP and NIP were -16.5 mV and -18.9 mV, respectively (Fig. 4a c). This means that the hydrodynamic surface charge of both polymers was negative, which also explains high adsorption of templates onto both MIP and NIP surface that was observed previously especially, at low pH.19 It has been reported that the maximum adsorption of target compounds onto the surface of the MIP takes place in acidic conditions where the analytes are protonated.11,19 MIP that was loaded with all target compounds at pH 2.5 gave a zeta potential of 0 mV (Fig. 4b). This phenomenon is depicted schematically in Fig. 5, which shows the orientation of adsorbed compounds on the surface of MIP molecule. In this figure, a clear picture of the protonated templates which are attached via their carboxyl end is demonstrated. In this case, the pH control played a significant role on the retention of these acidic molecules on the surface of the MIP. This phenomenon implied that the acidic molecules in their ionized forms (which can be obtained at high pH) would have been less retained by the MIP. However, this can be reversed by lowering the pH of the system which led to high retention of the acids. Further to this, it was previously explained that the hydrogen bonding interactions takes place between the carboxylic groups of target compounds and the nitrogen atom of the functional monomer, as shown in previous work.28

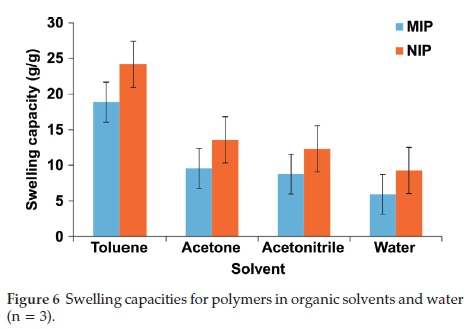
From swelling analysis, it was discovered that the swelling of the MIP was less when compared to the NIP (Fig. 6). This trend was observed in organic solvents (with different polarities) and water. The swelling trend followed the polarity order of toluene>acetone>acetonitrile>water. Swelling of the MIP could result in variations in the binding site cavities. As documented elsewhere, depending on the solvent used in the adsorption of target compounds, the MIP particles would increase in size (diameter) due to swelling which is turn could cause variations in the binding site cavities.32 This could ultimately alter the arrangement of functional groups of the MIP, and lead to a loss of recognition by affecting interactions between the target compounds and the polymer.32 This was evident in a previous study, where the recognition abilities of multi-template MIPs varied in different organic solvents.28 Therefore, lower swelling capacity for the MIP could also lead to stronger molecular recognition of template molecules.
3.2. Application
3.2.1. Validation of Analytical Method
The analytical conditions resulted in well-resolved peaks for target compounds and short analysis times (Fig. 7a,b). LOD, LOQ, accuracy, precision and linearity of the calibration curves were used to assess the validity of the analytical method (Table 2). For this validation, deionized water, raw water from DWTP, WWTP effluent and river water were spiked with all target compounds at a concentration of 5μgL-1. Chromatograms obtained for spiked and un-spiked samples are presented in Fig. 7c-f). LOD and LOQ were defined as the concentrations that produced the signal to noise ratio of 3 and 10, respectively. Based on LODs and LOQs, the sensitivity of the current method compared well with the results in literature for the HPLC quantification of the same compounds using photo diode array detection.7,33 The sensitivity of the current method can be improved by increasing sample volume; however, such work can lead to longer analysis time. It was further observed that the analytical method was highly accurate and precise due to high percentage recovery and low standard deviations. External calibration curves were linear for all the target compounds with R2 in the range of 0.994 to 0.999.
3.2.2. Analysis of Naproxen, Ibuprofen and Diclofenac in Water Resources
In most cases for WWTPs, the detected concentrations for the three compounds were below the quantification limits (Table 3). The most concentrated compound in wastewater samples was diclofenac with the maximum concentration of 1.44 ± 0.29 MgL-1 in the effluent. Previously, the relative abundance of ibuprofen and diclofenac reported in water systems in South Africa were 0.81 and 2.16 L-1, respectively.24
In comparison with concentrations reported in various South African WWTPs, the levels of target compounds detected in this study were generally lower.11,26,34,35 This could be due to the larger population groups served by various WWTPs reported in previous studies. Seasonal changes and variations in sample collection times (morning vs afternoon) could also influence the results.27 Further to this, previous studies focused more on the WWTPs that are located in the metropolitan areas (Johannesburg and Durban). From previous studies, it was observed that in South African major metropolitan cities, there are large quantities of naproxen, ibuprofen and diclofenac that enter the WWTPs. This observation is based on large quantities of such drugs that have been detected in WWTP influents collected in Durban and Johannesburg.26,36,37 The information on the occurrence of such drugs in water resources of small towns like Ladysmith is still missing in literature. Recent work in South Africa showed that these pharmaceutical drugs are not completely removed during the wastewater treatment process, therefore they are discharged into the rivers by various WWTPs.36,37 In this instance, the concentration ranges reported for diclofenac and ibuprofen in WWTP located in the Msunduzi district in Pietermaritzburg which is a less populated area when compared to Johannesburg and Durban were 12.4 to 22.3 L-1 and 1.06 to 1.38 L-1, respectively.25 As in the case of the results shown in Table 3, previous work showed that the concentrations for diclofenac in wastewater are generally higher than that of ibuprofen.25 Poor removal efficiency for diclofenac in Ladysmith WWTP was observed. In literature, there is evidence of poor removal efficiency of diclofenac during the wastewater treatment process.9,20 In relation to African studies, diclofenac concentrations were similar to those obtained in wastewater analysis of Algeria.20 In the same study 20, the concentrations of naproxen and diclofenac did not exceed 10 L-1 in wastewater samples. To some extent, the concentrations in wastewater were also similar to those obtained in certain European countries.38 For instance, the median concentrations reported in the influent of a WWTP located in the Spanish Mediterranean area of Valencia for naproxen, ibuprofen and diclofenac were 1.32,14.6 and 0.53 L-1, respectively.38 Ibuprofen was not detected in effluent; however, diclofenac and naproxen were found at median concentrations of 0.34 and 0.13 L-1, respectively.38
Surprisingly, the concentrations of the three compounds in certain river water samples, especially in upstream regions, were higher than those obtained in wastewater influent and effluent samples. This is probable due to poor sanitation and lack of WWTPs in this region which could lead to direct contamination of surface water with pharmaceutical drugs. The town of Ladysmith is mostly surrounded by rural areas with free grazing of animals such as cattle and goats along the river. At the time of sampling especially in upstream regions of the river, a lot of solid materials (such as plastic, paper, etc) were floating in the river, indicating direct disposal of solid waste into the river. The reduction of pharmaceutical concentrations downstream in the river might be due to the dilution effect. Target compounds were previously detected in other South African rivers 11,2434 which causes serious concerns to the general public regarding the status of these pollutants. It has already been reported that, in a few cases, the concentration of pharmaceuticals in South African surface water can exceed those obtained in wastewater.35 Globally, various concentrations of acidic pharmaceuticals have been reported in river water. In this regard, the maximum concentrations reported for naproxen, ibuprofen and diclofenac in Lis River (Portugal) were 260,1317 and 38 ng L-1.39 This indicates that pharmaceuticals cause serious water pollution all over the world. It has been reported that the continuous discharge of these pharmaceutical drugs have contributed to their persistence in the environment.40 Further to this, the comparison relating the concentrations obtained in this work with previous studies is given in Table 4. It is worth noting that, mostly the environmental monitoring of these drugs is carried out in urban areas and there is lack of available data for the occurrence of these compounds in rural waters.
In Europe, the three target compounds have been detected in low ng L-1 levels in drinking water9,16 which could indicate that the sensitivity of the proposed method needs further improvement which could be a separate study altogether. The levels of pharmaceuticals reported in South African water bodies are generally higher than those reported in other countries. Therefore, the proposed method was expected to detect the pharmaceuticals present in all samples. However, none of the three compounds were detected in DWTP samples. A more sensitive method is required to further investigate the occurrence of naproxen, ibuprofen and diclofenac in DWTP samples.
3.3. Water Quality at the Studied Sites
In general, the water quality of the studied sites was evaluated based on the common physical-chemical parameters. The results (Table 5) obtained compared well with water quality parameters of other African water bodies.41-43 According to South African National Standards, the pH permissible limit is 5 £ pH £ 9.7, pH units, while conductivity should not exceed 1700 cm-1 in drinking water.43 The increase of conductivity from the influent to the effluent suggests poor performance of the WWTPs. This was also evident for river water in downstream regions which could have been caused by the introduction of WWTP effluents into the surface water. In a previous study,41 a mean concentration of 8.14 mg L-1 for dissolved oxygen (DO) was obtained in river water which corresponds well with the results of the current study. Both low salinity and total dissolved solids (TDS) indicate that the quality of water was fairly good. An increase in TDS from wastewater influent to effluent was observed, this trend has been reported for a WWTP in Kenya.44
4. Conclusions
In the present study, the selective solid-phase extraction method for naproxen, ibuprofen and diclofenac was applied in the identification and quantification of these acidic drugs from wastewater, DWTP and river water samples. Multi-template molecularly imprinted polymer was prepared and used to enhance the selectivity of the analytical method. The recoveries, detection and quantification limits were determined in order to validate the analytical method. The analytical method was rapid due to the small sample volume (50 mL) percolated onto MISPE and chromatographic separation that was attained in less than 10 min. Furthermore, the analytical method was easy, selective, affordable and sensitive. Target compounds were not detected in DWTP samples; however, all compounds were detected in river water with ibuprofen being the most abundant pharmaceutical drug with maximum concentration of 6.72 μg L-1. Mostly, the concentrations of compounds in wastewater samples were below the limit of quantification except for diclofenac. Poor performance of WWTPs was evident during the analysis of physicochemical properties such as conductivity which demands the urgent need for the upgrade of these facilities. As this study is based on the initial assessment regarding the occurrence of naproxen, ibuprofen and diclofenac in Ladysmith water resources, more analyses need to be conducted in order to understand the full extent of pollution. Since traces of target compounds were detected, further work in the development of materials such as polymers for the adsorption of such compounds from the environment is required.
Acknowledgements
This work was supported by the National Research Foundation (NRF) of South Africa, Unique Grant No. 93986, and Eskom through Tertiary Education Support Program. NRF is also thanked for the funds allocated for lecturer replacement of L.M.M.. Silindile Zunngu and Siyabonga Mntambo (both MSc students) are acknowledged for zeta potential characterization and sample collection, respectively.
References
1 D.M. Pavlovic, S. Babic, A.J.M. Horvat and M. Kastelan-Macan, Sample preparation in analysis of pharmaceuticals, TrAC-Trend Anal. Chem., 2007, 26, 1062-1075. [ Links ]
2 M.R. Payan, M.A. Bello Lopez, R. Fernandez-Torres, J.L. Perez Bernal and M.C. Mochon, HPLC determination of ibuprofen, diclofenac and salicylic acid using hollow fiber-based liquid phase micro-extraction (HF-LPME), Anal. Chim. Acta, 2009, 653, 184-190. [ Links ]
3 A. Sarafraz-Yazdi, A. Amiri, G. Rounaghi and H. Eshtiagh-Hosseini, Determination of non-steroidal anti-inflammatory drugs in water samples by solid-phase microextraction based sol-gel technique using poly(ethylene glycol) grafted multi-walled carbon nanotubes coated fiber, Anal. Chim. Acta, 2012, 720, 134-141. [ Links ]
4 S. Tanwar, M. Di Carro and E. Magi, Innovative sampling and extraction methods for the determination of nonsteroidal anti-inflammatory drugs in water, J. Pharmaceut. Biomed., 2015 106, 100-106. [ Links ]
5 P. Paiga and C. Delerue-Matos, Determination of pharmaceuticals in groundwater collected in five cemeteries' areas (Portugal), Sci. Total Environ., 2016, 569-570, 16-22. [ Links ]
6 N. Lindqvist, T. Tuhkanen and L. Kronberg, Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters, Water Res., 2005, 39, 2219-2228. [ Links ]
7 J.L. Santos, I. Aparicio, E. Alonso and M. Callejon, Simultaneous determination of pharmaceutically active compounds in wastewater samples by solid phase extraction and high-performance liquid chromatography with diode array and fluorescence detectors, Anal. Chim. Acta, 2005, 550, 116-122. [ Links ]
8 N. Migowska, M. Caban, P. Stepnowski and J. Kumirska, Simultaneous analysis of non-steroidal anti-inflammatory drugs and estrogenic hormones in water and wastewater samples using gas chromatography-mass spectrometry and gas chromatography with electron capture detection, Sci. Total Environ., 2012,441, 77-88. [ Links ]
9 E. Carmona, V. Andreu and Y. Pico, Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: from waste to drinking water, Sci. Total Environ., 2014, 484, 53-63. [ Links ]
10 N. Gilart, R.M. Marce, N. Fontanals and F. Borrull, A rapid determination of acidic pharmaceuticals in environmental waters by molecu-larly imprinted solid-phase extraction coupled to tandem mass spectrometry without chromatography, Talanta, 2013,110, 196-201. [ Links ]
11 L.M. Madikizela, and L. Chimuka, Determination of ibuprofen, naproxen and diclofenac in aqueous samples using a multi-template molecularly imprinted polymer as selective adsorbent for solid-phase extraction, J. Pharmaceut. Biomed., 2016,128, 210-215. [ Links ]
12 T. Martinez-Sena, S. Armenta, M. de la Guardia and F.A. Esteve-Turrillas, Determination of non-steroidal anti-inflammatory drugs in water and urine using selective molecular imprinted polymer extraction and liquid chromatography, J. Pharmaceut. Biomed., 2016, 131, 48-53. [ Links ]
13 C. Dai, J. Zhang, Y. Zhang, X. Zhou, Y. Duan and S. Liu, Selective removal of acidic pharmaceuticals from contaminated lake water using multi-templates molecularly imprinted polymer, Chem. Eng. J., 2012, 211-212, 302-309. [ Links ]
14 A. Sarafraz-Yazdi and N. Razavi, Application of molecularly-imprinted polymers in solid-phase microextraction techniques, TrAC-Trend Anal. Chem., 2015, 73, 81-90. [ Links ]
15 P. Lacina, L. Mravcova and M. Vavrova, Application of comprehensive two-dimensional gas chromatography with mass spectrometric detection for the analysis of selected drugresidues in wastewater and surface water, J. Environ. Sci., 2013, 25, 204-212. [ Links ]
16 M. Caban, E. Lis, J. Kumirska and P. Stepnowski, Determination of pharmaceutical residues in drinking water in Poland using a new SPE-GC-MS(SIM) method based on Speedisk extraction disks and DIMETRIS derivatization, Sci. Total Environ., 2015, 538, 402-411. [ Links ]
17 L.M. Madikizela, S.F. Muthwa and L. Chimuka, Determination of triclosan and ketoprofen in river water and wastewater by solid phase extraction and high performance liquid chromatography, S. Afr. J. Chem., 2014, 67, 143-150. [ Links ]
18 L. Patrolecco, N. Ademollo, P. Grenni, A. Tolomei, A.B. Caracciolo and S. Capri, Simultaneous determination of human pharmaceuticals in water samples by solid phase extraction and HPLC with UV-fluo-rescence detection, Microchem. J., 2013,107, 165-171. [ Links ]
19 L.M. Madikizela and L. Chimuka, Synthesis, adsorption and selectivity studies of a polymer imprinted with naproxen, ibuprofen and diclofenac, J. Environ. Chem. Eng., 2016, 4, 4029-4037. [ Links ]
20 A.E.B. Kermia, D. Fouial-Djebba and M. Trari, Occurrence, fate and removal efficiencies of pharmaceuticals in wastewater treatment plants (WWTPs) discharging in the coastal environment of Algiers, C. R. Chim., 2016,19, 963-970. [ Links ]
21 J. Debska, A. Kot-Wasik and J. Namiesnik, Fate and analysis of pharmaceutical residues in the aquatic environment, Crit. Rev. Anal. Chem., 2004, 34, 51-67. [ Links ]
22 T.E. Felix-Canedo, J.C. Duran-Alvarez, and B. Jimenez-Cisneros, The occurrence and distribution of a group of organic micropollutants in Mexico City's water sources, Sci. Total Environ., 2013, 454-455, 109-118. [ Links ]
23 Y. Duan, C. Dai, Y. Zhang and L. Chen, Selective trace enrichment of acidic pharmaceuticals in real water and sediment samples based on solid-phase extraction using multi-templates molecularly imprinted polymers, Anal. Chim. Acta, 2013, 758, 93-100. [ Links ]
24 F.O. Agunbiade and B. Moodley, Pharmaceuticals as emerging organic contaminants in Umgeni River water system, KwaZulu-Natal, South Africa, Environ. Monit. Assess., 2014,186, 7273-7291. [ Links ]
25 F.O. Agunbiade and B. Moodley, Occurrence and distribution pattern of acidic pharmaceuticals in surface water, wastewater, and sediment of the Msunduzi river, KwaZulu-Natal, South Africa, Environ. Toxicol. Chem., 2016, 35, 36-46. [ Links ]
26 R. Amdany, L. Chimuka and E. Cukrowska, Determination of naproxen, ibuprofen and triclosan in wastewater using the polar organic chemical integrative sampler (POCIS): a laboratory calibration and field application, Water SA, 2014, 40, 407-414. [ Links ]
27 R. Amdany, A. Moya, L. Chimuka and E. Cukrowska, Optimization of the temperature for the extraction of pharmaceuticals from wastewater by a hollow fiber silicone membrane, Anal. Lett., 2015,48, 2343-2356. [ Links ]
28 L.M. Madikizela, P.S. Mdluli and L. Chimuka, Experimental and theoretical study of molecular interactions between 2-vinyl pyri-dine and acidic pharmaceuticals used as multi-template molecules in molecularly imprinted polymer, React. Funct. Polym., 2016, 103, 33-43. [ Links ]
29 I. Kaur, S. Rattan, S. Chauhan and N. Gupta, Gamma-radiation-induced grafting of binary mixture of methacrylic acid and 4-vinyl pyridine onto Teflon-FEP film as an effective polar membrane for separation processes, Nucl. Instrum. Methods B, 2010,268,1642-1652. [ Links ]
30 E. Lutge, N. Moodley, A. Tefera, B. Sartorius, T. Hardcastle and D. Clarke, A hospital based surveillance system to assess the burden of trauma in KwaZulu-Natal Province South Africa, Injury, 2016, 47, 135-140. [ Links ]
31 Z. Terzopoulou, M. Papageorgiou, G.Z. Kyzas, D.N. Bikiaris and D.A. Lambropoulou, Preparation of molecularly imprinted solid-phase microextraction fiber for the selective removal and extraction of the antiviral drug abacavir in environmental and biological matrices, Anal. Chim. Acta, 2016, 913, 63-75. [ Links ]
32 K. Farrington and F. Regan, Investigation of the nature of MIP recognition: the development and characterisation of a MIP for ibuprofen, Biosens. Bioelectron., 2007, 22, 1138-1146. [ Links ]
33 M.R., Payan, M.A. Bello Lopez, R.F. Torres, M.V. Navarro and M.C. Mochon, Electromembrane extraction (EME) and HPLC determination of non-steroidal anti-inflammatory drugs (NSAIDs) in wastewater samples, Talanta, 2011, 85, 394-399. [ Links ]
34 S. Matongo, G. Birungi, B. Moodley and P. Ndungu, Pharmaceutical residues in water and sediment of Msunduzi River, KwaZulu-Natal, South Africa, Chemosphere, 2015, 134, 133-140. [ Links ]
35 S. Matongo, G. Birungi, B. Moodley and P. Ndungu, Occurrence of selected pharmaceuticals in water and sediment of Umgeni River, KwaZulu-Natal, South Africa, Environ. Sci. Pollut. Res., 2015, 22, 10298-10308. [ Links ]
36 L.M. Madikizela, and L. Chimuka, Occurrence of naproxen, ibuprofen, and diclofenac residues in wastewater and river water of KwaZulu-Natal province in South Africa, Environ. Monit. Assess., 2017, 189, 348-359. [ Links ]
37 L.M. Madikizela, and L. Chimuka, Simultaneous determination of naproxen, ibuprofen and diclofenac in wastewaterusingsolid-phase extraction with high performance liquid chromatography, Water SA, 2017, 43, 264-274. [ Links ]
38 E. Gracia-Lor, J.V. Sancho, R. Serrano and F. Hernandez, Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia, Chemosphere, 2012, 87, 453-462. [ Links ]
39 P. Paiga, L.H.M.L.M. Santos, S. Ramos, S. Jorge, J.G., Silva and C. Delerue-Matos, Presence of pharmaceuticals in the Lis river (Portugal): sources, fate and seasonal variation, Sci. Total Environ., 2016,573, 164-177. [ Links ]
40 X. Yuan, Z. Qiang, W. Ben, B. Zhu, and J. Liu, Rapid detection of multiple class pharmaceuticals in both municipal wastewater and sludge with ultra high performance liquid chromatography tandem mass spectrometry, J. Environ. Sci., 2014, 26, 1949-1959. [ Links ]
41 S. Munyika, V. Kongo, and R. Kimwaga, River health assessment using macroinvertebrates and water quality parameters: a case of the Orange River in Namibia, Phys. Chem. Earth, 2014, 76-78, 140-148. [ Links ]
42 E.M.M. Wanda, L.C. Gulula, and G. Phiri, Determination of characteristics and drinking water quality index in Mzuzu City, Northern Malawi, Phys. Chem. Earth, 2012, 50-52, 92-97. [ Links ]
43 E.M.M. Wanda, B.B. Mamba and T.A.M. Msagati, Determination of the water quality index ratings of water in the Mpumalanga and North West provinces, South Africa, Phys. Chem. Earth., 2016, 92, 70-78. [ Links ]
44 K.O. K'oreje, L. Vergeynst, D. Ombaka, P. De Wispelaere, M. Okoth, H. Van Langenhove and K. Demeestere, Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya, Chemosphere, 2016, 149, 238-244. [ Links ]
Received 4 November 2016
Revised 16 August 2017
Accepted 4 October 2017.
* To whom correspondence should be addressed. E-mail: lawrencem2@dut.ac.za















