Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840XPrint version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.78 Durban 2024
https://doi.org/10.17159/0379-4350/2024/v78a17
RESEARCH ARTICLE
Synthesis and docking studies of 1,3,4-oxadiazole, keto pyrazole, pyrrole and lactam derivatives of Thienopyrimidinesas EGFR tyrosine kinase inhibitors
Giri TharikoppulaI; Shashikala KethireddyII; Suresh BairiIII; Murali MalleliII; Laxminarayana EppakayalaI,
ISreenidhi Institute of Science and Technology (Autonomous) Yamnampet, Ghatkesar, Hyderabad, India
IIGeethanjali College of Engineering and Technology, Keesara, Rangareddy, India
IIIPalamuru University, Bandameedipally, Mahbubnagar, Telangana, India
ABSTRACT
Derivatives of thieno[3,2-d]pyrimidines were synthesized from a starting material 4-(4-(methylamino)thieno[3,2-d]pyrimidin-2-yl) benzohydrazide and thoroughly characterized by using various spectroscopic techniques such as Infra Red, 1H Nuclear Magnetic Resonance, 13C Nuclear Magnetic Resonance and Mass spectrometry. The study aimed to explore the potential of these derivatives as anti-cancer agents targeting the epidermal growth factor receptor (EGFR), a primary target in cancer development. The synthesized compounds underwent molecular docking using Auto dock tools to assess the interaction with EGFR. The EGFR tyrosine kinase obtained from the Protein Data Bank was used for docking studies. Among all the four compounds subjected to docking, compound 3 exhibited the highest binding energy (-10.73 kcal/mol) with EGFR.
Keywords: Thienopyrimidines, EGFR, Anti-cancer activity, Docking, Protein kinases
INTRODUCTION
Cancer is a global health problem that is increasingly prevalent and leading to high mortality rates. The development and progression of cancer cells involve the activity of certain enzymes known as protein kinases.1 As a result, protein kinase inhibitors gained significant importance in cancer treatment, targeting pathways that address various cellular communication issues. Protein kinases are a prime focus in clinical oncology due to their pivotal role in signal transduction pathways, contributing to metastasis and drug resistance. The development of kinase inhibitors as anticancer drugs remains key research area to improve selectivity, safety and efficacy.
Among the chemical scaffolds utilized in drug development, the thien-opyrimidine scaffold is widely employed. Thienopyrimidine-containing compounds share structural and isoelectronic characteristics with purines,1 making them attractive for pharmaceutical drug production. Thienopyrimidines have demonstrated diverse pharmacological properties, including anti-bacterial,2,3 anti-viral, anti-cancer,4,5 anti-fungal,6,7 anti-inflammatory and anti-protozoal activities.8,9 Figure 1 illustrates several thienopyrimidine-containing drugs with varying biological activity profiles.
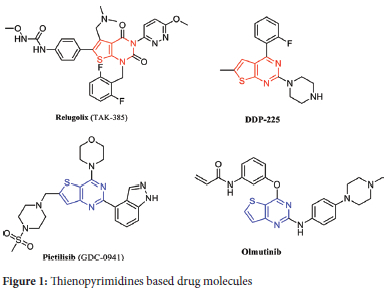
For instance, Relugolix (TAK-385) has potential to treat prostate carcinoma; DDP-225 aims to treat gastrointestinal tract diseases and irritable bowel syndrome. Pictilisib (GDC-0941) is used to treat advanced solid tumors. Additionally, Olmutinib is an approved drug that inhibits epidermal growth factor receptor (EGFR) and is utilized in the treatment of small cell lung cancer.
In summary, due to the escalating global impact of cancer, effective treatment strategies and early diagnosis are crucial. Inhibiting protein kinases and other relevant targets have become essential approaches in cancer treatment. Thienopyrimidine drugs with their diverse pharmacological properties have shown promise in the development of various drugs, especially, anti-cancer drugs.
Scheme 1 outlines the synthesis of several compounds using different reagents and conditions. Compound 1 was treated with triethyl orthoformate (2) in the presence of p-TSA (p-toluene sulfonic acid),
in ethanol solvent at 90oC for 1 hour under microwave irradiation to afford pure target compound 3. Compound 1 was reacted with pentane-2,4-dione 4(1,3-diketone) in the presence of P-TSA in ethanol solvent at 90oC for 1hour under microwave irradiation to achieve pure target compound 5. Compoundl was also treated with hexane-2,5-dione (6) in the presence of P-TSA in ethanol solvent at 90oC for 1 hour under microwave irradiation to give compound 7. Further, Compound 1 was condensed with 4-chlorobenzaldehyde (8) in the presence of P-TSA in ethanol solvent at 90oC for 1hour under microwave irradiation to afford pure target compound 9. Finally, compound 9 was reacted with chloroacetyl chloride (10) in DMF (dimethyl formamide) solvent and was heated at 120 oC for 24 hours to afford pure target compound 11.
The reactions use p-TSA as a catalyst and microwave irradiation to accelerate the reaction and reduce the reaction time. The solvents used are ethanol and DMF. The temperatures and reaction times are also specified for each step.
EXPERIMENTAL
All solvents and chemicals were obtained commercially, from Sigma-Aldrich and used without further purification. All the reactions were monitored by TLC on Merck Kieselgel 60 F524, by UV light and/ or spraying a 5% H2SO4 in Ethanol followed by heating. Column chromatography was performed on Silica Gel 60 (60-120 mesh). Melting points were determined in open capillary tubes on a GallenKamp MFB-595 apparatus. Element analysis was carried out with Thermofinnigan CHNS analyzer. IR spectra were recorded with Shimadzu FTIR 8400s spectrophotometer. 1H NMR spectra were recorded on a Bruker 500 MHz NMR spectrometer. Mass spectra were recorded by Shimadzu mass spectrometer.
General procedure for Synthesis of 1,3,4-oxadiazole, keto pyrazole, pyrrole and hydrazone derivatives of Thienopy-rimidines (3,5,7&9)
To a solution of 4-(4-(methylamino)thieno[3,2-d]pyrimidin-2-yl) benzohydrazide (1)(100 mg, 0.33 mmol) in ethanol (4 mL) was added corresponding tri ethyl orthoformate or diketone or aldehyde (0.36 mmol) followed by p-TSA (catalyst) and irradiated to 90 0C for 1h in microwave oven. After the completion, reaction mixture was poured into ice water (4 mL), filtered and the obtained precipitate was washed with ethyl acetate to afford compounds 3,5,7 & 9.
2-(4-(1,3,4-oxadiazol-2-yl)phenyl)-N-methylthieno[3,2-d] pyrimidin-4-amine (3)
Yield: 91%; Off white sold; m.p: 220-225oC; IR (KBr): umax 3440, 3314, 3082, 1665, 1601, 1539, 1389, 1345, 1182, 1110, 1039, 949, 902, 858, 793, 712, 645, 563, 527;1H NMR (500 MHz, DMSO): δ 9.401 (s, 1H), 8.657-8.640 (d, J = 8.5 Hz, 2H), 8.182-8.166 (d, J = 8 Hz, 4H), 7.5037.492 (d, J = 5.5 Hz, 1H), 3.152-3.143 (d, J = 4.5 Hz, 3H);ESI-MS: m/z, 310.11 (M+H)+;
(3,5-dimethyl-1H-pyrazol-1-yl)(4-(4-(methylamino)thien-o[3,2-d]pyrimidin-2-yl)phenyl)methanone (5)
Yield: 88%;off white sold; m.p: 222-227oC; IR (KBr): umax3270, 3151, 3064, 2930, 2890, 1927, 1812, 1708, 1597, 1541, 1504, 1427, 1384, 1336, 1269, 1189, 1044, 970, 908, 857, 788, 704, 658, 609, 568, 529; 1H NMR (400 MHz, DMSO): δ 8.578-8.557 (d, J = 8.4 Hz, 2H), 8.156-8.142 (d, J = 5.6 Hz, 1H), 7.993-7.972 (d, J = 8.4 Hz, 3H), 7.501-7.488 (d, J = 5.21 Hz, 1H), 6.307 (s, 1H), 3.142-3.131 (d, J = 4.4 Hz, 3H), 2.591 (s, 3H), 2.183 (s, 3H); 13C NMR (500 MHz, DMSO): δ 13.47-13.87, 27.24, 111.327, 113.932, 124.686, 126.932, 130.795, 133.196, 134.126, 141.975, 144.429, 151.697, 157.383, 158.787, 159.887, 167.731; ESI-MS: m/z, 364.24 (M+H)+;
N-(2,5-dimethyl-1H-pyrrol-1-yl)-4-(4-(methylamino)thien-o[3,2-d]pyrimidin-2yl)benzamide(7)
Yield: 91%;Off white sold; m.p: 214-219oC; IR (KBr): umax 3364, 3263, 3188, 2923, 2857, 1814, 1731, 1674, 1620, 1598, 1535, 1497, 1388, 1349, 1271, 1172, 1116, 1038, 1011, 905, 760, 690, 647, 565; 1H NMR (500 MHz, DMSO): 511.377 (s, 1H), 8.573-8.557 (d, J = 8 Hz, 2H), 8.223-8.213 (d, J = 5 Hz, 1H), 8.114-8.098 (d, J = 8 Hz, 2H), 7.5197.508 (d, J = 5.5 Hz, 1H), 7.480-7.464 (d, J = 8 Hz, 1H), 7.116-7.101 (d, J = 7.5 Hz, 1H), 5.729 (s, 2H), 3.168 (s, 3H), 2.286 (s, 1H), 2.067 (s, 6H);ESI-MS: m/z, 378.28 (M+H)+.
Synthesis of (E)-N'-(4-chlorobenzylidene)-4-(4-(methylami-no)thieno[3,2-d]pyrimidin-2-yl)benzohydrazide (9)
Yield: 87%; White solid;m.p: 282-290oC; IR (KBr): umax3332, 3242, 3052, 2928, 2856, 1649, 1582, 1535, 1492, 1388, 1347, 1275, 1047, 822, 652,cm-1; 1H NMR (400 MHz, DMSO): δ 12.01 (s,1H), 8.59-8.49 (m, 3H), 8.14-8.13 (d, J = 5.2 Hz, 1H), 8.052-8.032 (d, J = 8 Hz, 2H), 7.9637.952 (d, J = 4.4 Hz, 1H), 7.795-7.775 (d, J = 8 Hz, 2H), 7.556-7.483 (m, 3H), 3.14-3.13 (s, J = 4.4 Hz, 3H); 13C NMR (400 MHz, DMSO): 18.5, 27.2, 56.0 113.8, 124.6, 127.5-127.7, 128.4-128.9, 133.1-133.4, 134.3-134.6, 141.4, 146.4, 157.3, 158.9, 159.9, 163.2; ESI-MS: m/z, 422.2 (M+H)+; HRMS (ESI): m/z calcd for C21H17N5OSCl[M + H]+: 422.0842; found: 422.0825.
N-(2-chloro-3-(4-chlorophenyl)-4-oxoazetidin-1-yl)-4-(4-(methylamino)thieno[3,2-d]pyrimidin-2-yl)benzamide (11)
To a stirred solution of (E)-N'-(4-chlorobenzylidene)-4-(4-(methyl-amino)thieno[3,2-d]pyrimidin-2-yl)benzohydrazide (9) (100 mg, 1eq.) in DMF (2 mL) was added TEA (2 eq.), chloro acetylchloride 10 (10 eq.) as drop wise and heated to 120°C for 36 h. After completion, solvent was evaporated to get crude residue. crude residue was poured in to ice water basified withaq.Na2CO3 solution, extracted with 10% MeOH in DCM (10 mL X 3 Times). Combined extracts were washed with water followed by brine solution, dried the organics over anhydrous Na2SO4 filtered and evaporated to get the crude product. The crude product was purified by column chromatography eluted with 8% MeOH in DCM to afford 11 as yellow solid. Yield: 87%; pale yellow sold; m.p: 228-233oC;1H NMR (500 MHz, DMSO): δ 8.6078.590 (d, J = 6.5 , 2H), 8.145-8.134 (d, J=5.5 ,1H), 8.134-7.973 (d, J=8 ,2H), 7.959 (s, 1H), 7.956-7.934 (m, J=5.5 ,4H), 7.485-7.475 (d, J=5 ,2H), 7.301 (s, 1H), 4.779-4.751 (d, J=1.5 ,1H), 4.673-4.644 (d, J=1.5 ,1H), 3.119 (s, 3H); 13C NMR (500 MHz, CDCl3): δ 27.26, 28.96, 41.89, 91.50, 113.94, 124.35-124.59 126.84, 128.22, 128.72-128.98, 133.29, 134.71-134.82, 155.32, 157.37, 158.53, 162.61;ES1-MS: m/z, 498.10 (M+H)+.
RESULTS AND DISCUSSION
Molecular docking studies
Molecular docking investigations were conducted using Auto dock tools focusing on the epidermal growth factor receptor (EGFR) as the primary target. EGFR protein, a cell-surface receptor, is a crucial target for the development of anti-cancer agents10 and plays a significant role in the ductal development of the mammary gland.11 Over expression of EGFR is associated with various cancers.12 The protein, with the PDB 1D 4HJOEGFR tyrosine kinase, was obtained from the Protein Data Bank.13,14
Among the four compounds subjected to docking, compound 3 exhibited the highest binding energy measuring -10.73 kcal/mol with EGFR. 1t formed a single hydrogen bond with the amino acid residue LEU838, with bond length 1.95 Â. Further, compound 3 exhibitedn-n stacking with the PHE699 residue. Compound 1 displayed second highest binding energy (-9.51 kcal/mol) and formed a hydrogen bond with the ASP831 amino acid residue, with a bond length of 2.15 Â. Similarly, nitrogen atom of amide bond in compound 4 established ahydrogen bond with THR830 residue, measuring bond length of 1.96 Â. Lastly, compound 2 has exhibited the lowest binding energy (-7.59 kcal/mol) among the four compounds and formed two hydrogen bonds. The first bond had a bond length of 1.96 7Â with LYS721 residue while the second bond had a bond length2.13 Â respectively with ASP831 residue (Figure 2-5 and Table 1).
CONCLUSION
A series of derivatives including 1,3,4-oxadiazole, keto pyrazole, pyrrole and hydrazone derivatives of thieno[3,2-d]pyrimidines were successfully synthesized and purified using column chromatography.
Their melting points were determined to assess their purity. Additionally, elementary analysis was performed on all four final compounds to determine the elemental composition accurately. To further characterize the synthesized products, spectroscopic techniques such as IR, 1H NMR, 13C NMR and Mass were employed.
In pursuit of potential anti-cancer agents, the binding affinity of these derivatives was also investigated using molecular docking through Auto dock tools with a focus on the epidermal growth factor receptor (EGFR) which plays a pivotal role in cancer development. The study helps in the development of new anti-cancer agents. Among the four compounds subjected to docking, compound 3 exhibited the highest binding energy measuring -10.73 kcal/mol with EGFR.
ORCID IDS
Shashikala Kethireddy: https://orcid.org/0000-0001-6160-6434
Suresh Bairi: https://orcid.org/0000-0002-8816-5832
Murali Malleli: https://orcid.org/0000-0002-0284-4309
Laxminarayana Eppakayala: https://orcid.org/0000-0003-4465-042X
REFERENCES
1. Sayed MTM, Hassan RA, Halim PA, El-Ansary AK. Recent updates on thienopyrimidine derivatives as anticancer agents. Med Chem Res. 2023;32(4):659-681. https://doi.org/10.1007/s00044-023-03040-y. [ Links ]
2. Ali EM, Abdel-Maksoud MS, Oh C-H, Abdel-Maksoud MS, Oh CH. Thieno [2,3-d] pyrimidine as a promising scaffold in medicinal chemistry Recent advances. Bioorg Med Chem. 2019;27(7):1159-1194. https://doi.org/10.1016/j.bmc.2019.02.044. [ Links ]
3. Giri T, Sailaja G, Laxminarayana E, Thirumala Chary M, Ramesh M. Synthesis and antibacterial activity of novel 4-{4-(methylamino) thieno [3,2-d] pyrimidin-2-yl}-benzohydrazide derivatives. Russ J Gen Chem. 2017;87(6):1275-1280. https://doi.org/10.1134/S1070363217060238. [ Links ]
4. Habib NS, Soliman R, El-Tombary AA, El-Hawash SA, Shaaban OG. Synthesis and biological evaluation of novel series of thieno [2,3-d] pyrimidine derivatives as anticancer and antimicrobial agents. Med Chem Res. 2013;22(7):3289-3308. https://doi.org/10.1007/s00044-012-0324-3. [ Links ]
5. Temburnikar KW, Zimmermann SC, Kim NT, Ross CR, Gelbmann C, Salomon CE, Wilson GM, Balzarini J, Seley-Radtke KL. Antiproliferative activities of halogenated thieno [3,2-d] pyrimidines. Bioorg Med Chem. 2014;22(7):2113-2122. https://doi.org/10.1016/j.bmc.2014.02.033. [ Links ]
6. Kanawade SB, Toche RB, Rajani DP. Synthetic tactics of new class of 4-aminothieno [2, 3-d] pyrimidine-6-carbonitrile derivatives acting as antimicrobial agents. Eur J Med Chem. 2013;64:314-320. https://doi.org/10.1016/j.ejmech.2013.03.039. [ Links ]
7. Ross CR, Temburnikar KW, Wilson GM, Seley-Radtke KL. Mitotic arrest of breast cancer MDA-MB-231 cells by a halogenated thieno [3,2-d] pyrimidine. Bioorg Med Chem Lett. 2015;25(8):1715-1717. https://doi.org/10.1016/j.bmcl.2015.02.071. [ Links ]
8. Shao X, AbdelKhalek A, Abutaleb NS, Velagapudi UK, Yoganathan S, Seleem MN, Talele TT. Chemical space exploration around thieno [3, 2-d] pyrimidin-4 (3 H)-one scaffold led to a novel class of highly active Clostridium difficile inhibitors. J Med Chem. 2019;62(21):9772-9791. https://doi.org/10.1021/acs.jmedchem.9b01198. [ Links ]
9. Aly HM, Saleh NM, Elhady HA. Design and synthesis of some new thiophene, thienopyrimidine and thienothiadiazine derivatives of antipyrine as potential antimicrobial agents. Eur J Med Chem. 2011;46(9):4566-4572. https://doi.org/10.1016/j.ejmech.2011.07.035. [ Links ]
10. McBryan J, Howlin J, Napoletano S, Martin F; McBryanJ. Howlin J, NapoletanoS,MartinF.Amphiregulin: role in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2008;13(2):159-169. https://doi.org/10.1007/s10911-008-9075-7. [ Links ]
11. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57-70. https://doi.org/10.1016/S0092-8674(00)81683-9. [ Links ]
12. Walker F, Abramowitz L, Benabderrahmane D, Duval X, Descatoire V, Hénin D, Lehy T, Aparicio T. Growth factor receptor expression in anal squamous lesions: modifications associated with oncogenic human papillomavirus and human immunodeficiency virus. Hum Pathol. 2009;40(11):1517-1527. https://doi.org/10.1016/j.humpath.2009.05.010. [ Links ]
13. Park JH, Liu Y, Lemmon MA, Radhakrishnan R. Erlotinib binds both inactive and active conformations of the EGFR tyrosine kinase domain. Biochem J. 2012;448(3):417-423. https://doi.org/10.1042/BJ20121513. [ Links ]
14. Xiao Z, Zhou Z, Chu C, Zhang Q, Zhou L, Yang Z, Li X, Yu L, Zheng P, Xu S, et al. Design, synthesis and antitumor activity of novel thiophene-pyrimidine derivatives as EGFR inhibitors overcoming T790M and L858R/T790M mutations. Eur J Med Chem. 2020;203:112511. https://doi.org/10.1016/j.ejmech.2020.112511. [ Links ]
Received 5 September 2023
Revised 19 February 2024
Accepted 8 March 2024
* To whom correspondence should be addressed: Email: elxnkits@yahoo.co.in













