Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.95 n.1 Pretoria 2024
https://doi.org/10.36303/JSAVA.583
ORIGINAL RESEARCH
Detection and quantification of antibiotic residues in goat milk in Mahikeng Local Municipality
KL NdlovuI; M MwanzaI, II; N NleyaI; L NgomaI
IDepartment of Animal Health, School of Agriculture, North-West University, South Africa
IIFood Security and Safety Niche Area, Faculty of Natural and Agricultural Sciences, North-West University, Mafikeng Campus, South Africa
ABSTRACT
Goat milk could be used to reduce malnutrition since it is highly nutritious, and many people in rural communities in South Africa rear small ruminants for survival. However, the risk of food contamination by antibiotic residues is one of the significant problems facing public health, and is a result of the irresponsible use of veterinary drugs. One hundred goat farmers were interviewed using a questionnaire, and raw milk samples from 266 goats were collected and analysed for the presence of antibiotic residues. Screening for amoxicillin, tetracycline, sulfamethazine, erythromycin, and streptomycin residues was done using the enzyme-linked immunosorbent assay (ELISA) while high-performance liquid chromatography (HPLC) was carried out for confirmation. The questionnaire shows that all (100%) of the participants acknowledged the use of antibiotics on their goats and 99% of them were aware of the possibility of antibiotic residues in milk. ELISA results for residues of erythromycin, sulfamethazine and amoxicillin exceeded the Codex Alimentarius maximum residue levels (MRLs) in 94.7%, 82.3%, and 35.3% of analysed samples, respectively. Tetracycline was present in all (100%) analysed milk samples, and streptomycin was detected in 18.7% of samples; however, these results were below the recommended MRLs. The HPLC method confirmed the presence of streptomycin and tetracycline residues in 90% and 40% of the samples analysed. However, the concentrations were below the accepted MRL standards. Approximately 76.6% of samples exceeded the established MRL for sulfamethazine and 10% for erythromycin. Amoxicillin was not detected by the HPLC method. The results obtained in this study indicate a high level of contamination of goat milk with antibiotic residues, which may harm the health of the consumers.
Keywords: antibiotic residues, goat milk, maximum residue level, screening method, confirmatory method
Introduction
Increasing poverty and exponential human population growth globally, including in South Africa, demand the need to investigate alternative sources of protein. The use of cheaply available animal protein by rural communities may help to alleviate hunger and confront food insecurity. There exists the possibility of using goat milk as a potential protein source and for income generation to improve the livelihoods of people, especially those in rural areas. The potential of goat milk as an ideal food source is irrefutable. Goat milk is richer in protein, vitamins, minerals, and calories than cow's milk (Bernacka 2011). Belewu & Adewole (2009) stated that due to the affordability, accessibility, and nutritional value of goat milk, it is preferable for consumption among people in rural communities compared to cow's milk. Even though goat milk is currently produced on a limited scale in South Africa, interest in the milking of goats has increased considerably over the past years (Peacock 2005).
However, animals in rural areas are mostly raised in low-quality fields with frequently infested feed and where minimal preventive measures (immunisation, concentrates, nutrients, etc.) are taken. Furthermore, they are usually kept in high densities clustered in areas/herds, under unsanitary conditions, and suffering from various infections, necessitating the administration of antibiotics. Antibiotics are generally safe drugs and helpful in fighting bacterial infectious diseases when used according to prescription (Eagar et al. 2012). Moreover, antibiotics are administered for prophylactic reasons, metaphylactic (control treatment), and growth-promoting purposes (Jank et al. 2015). According to Hollis & Ahmed 2013, approximately 80% of all antibiotics used globally are in agriculture and aquaculture.
The extensive use of antibiotics by farmers, combined with poor livestock production practices, such as failure to follow the guidelines (dose and withdrawal period) results in antibiotic residues often being found or detected in edible animal products, including milk, meat, and eggs (Lee et al. 2001; Hsieh et al. 2011). Governments and other stakeholder organisations have set up laws and guidelines for the use of veterinary medications to guarantee the safety and hygiene of foods of animal origin. It is therefore important to monitor the presence of residual drugs in livestock products at regular intervals, to evaluate whether the guidelines have resulted in effective food product safety and prevent and manage unforeseen challenges associated with this issue.
The presence of antibiotics in food for human consumption relates to many adverse public health effects, including gastrointestinal disturbances, hypersensitivity, tissue damage as well as neurological disorders (Ramatla et al. 2017). Antibiotic residues have also resulted in the development of antibiotic-resistant bacteria, leading to the decreasing efficacy of antibiotics in treating some infections (Bitas & Samanidou 2018). The analytical methods used for detecting antibiotic residues in food can be categorised into two groups: screening, and confirmatory methods (Ramatla et al. 2017). The screening methods include enzyme-linked immunosorbent assay (ELISA), thin layer chromatography (TLC), and the four plate tests (Adrian et al. 2009; Gaudin et al. 2004; Kaya & Filazi 2010). Methods based on high-performance liquid chromatography-mass spectrometry (HPLC-MS) detection are viewed as confirmatory methods since they give full or complementary data, enabling substances to be unequivocally recognised and, if necessary, quantified at the level of interest, as recommended by the European Commission Decision 2002/657/EC (Gavilán et al. 2016).
Research method and design
Study area
The study was conducted in Mahikeng, North-West Province, South Africa. The city shares an international border with the Republic of Botswana to the North and lies 260 km west of Johannesburg. Mahikeng is on the open veld at an elevation of 1 500 m along the banks of the Upper Molopo River. Climatic conditions differ significantly from west to east. The western region receives less than 300 mm of rain per annum, the central region around 550 mm per annum, while the eastern and south-eastern regions receive over 600 mm per annum (De Villiers & Mangold 2002). According to the local Department of Agriculture in Mahikeng, the area had 17 574 communal goats in 2018. The city has unimproved grasslands, and animals graze freely on natural rangeland. The vegetation type in all the areas resembles that of a savannah biome.
Sample collection
One hundred structured questionnaires were randomly distributed to farmers to collect data on possible use and risk associated with the administration of antibiotics, and 266 raw goat milk samples were collected from different goat breeds, including mixed breeds, from August to October 2018. The sample size required for the study was calculated using Epi-info software (Dean et al. 2000), assuming a 5% acceptance error, 50% expected prevalence, and confidence level of 90%. A total volume of 250 ml of milk sample was collected aseptically from both mammary glands of each selected clinically healthy goat into a sterile container. The samples were placed in a cooler box filled with ice and transported to the Animal Health Laboratory, North-West University, and stored at -70 °C until further analysis.
Antibiotic detection and quantification
ELISA method
The RIDASCREEN® ELISA test kit (Biopharm, Darmstadt, Germany) and ELABSCIENCE® ELISA test kit (USA) were used for screening antibiotic residues in milk samples. All the reagents required for the enzyme immunoassay, including standards, were included in the ELISA test kit. The RIDASCREEN® ELISA test kit was used to analyse tetracycline (Art: R3505), sulfamethazine (Art: R3001), and streptomycin (Art: R3104). The ELABSCIENCE® ELISA test kit was used for amoxicillin (catalogue No: E-FSE048) and erythromycin (catalogue No: E-FS-E060).
Sample extractions
The frozen milk samples were thawed at room temperature, and all necessary reagents were placed at room temperature (20-25 °C). The ELISA extraction process was performed according to the manufacturer's instructions. Then 50 μL of each standard solution was added in six different microtiter wells of the ELISA plate. The extracted samples were then individually added to the remaining 90 microtiter wells. The conjugated enzyme and antibody were added to the wells and mixed gently.
The resulting solution for amoxicillin, sulfamethazine, streptomycin, and erythromycin was incubated for 30 minutes, while for tetracycline the incubation period was one hour at room temperature (25 °C). Following incubation, the liquid was discarded, and the wells were washed three times with the wash buffer. A total of 100 μL of the substrate/chromogen was then added to the wells and incubated at room temperature for 15 minutes. After incubation, 100 μL of stop solution was added to the mixture.
The absorbance of the samples was then read at 450 nm, and the antibiotic residue concentrations were calculated based on the calibration curve of the standards. The reading was done within 30 minutes after adding the stop solution. The percentage (%) of absorbance was calculated using the following formula:
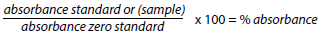
HPLC method
Sample extraction
The extraction of tetracycline, sulfamethazine, streptomycin, amoxicillin, and erythromycin residues in milk samples was done according to Abbasi et al. 2011, Gonzales et al. 2009, Andy 2013, Luo et al. 1997 and Clark et al. 2011, respectively.
Analysis
The analysis of tetracycline and sulfamethazine residues in milk samples was done using the fluorometric detection method (Cinquina et al. 2003; Su et al. 2008). Analysis of streptomycin, amoxicillin, and erythromycin residues was performed using a photodiode detector (PDA), according to Ashad et al. 2012, De Abreu et al. 2003 and García-Mayor et al. 2015, respectively. A total of 30 samples per antibiotic were analysed in the study.
Preparation of standard solutions and validation of HPLC techniques
Individual standards for the high-performance liquid chromatography (HPLC) analysis were prepared by dissolving 10 mg of each compound into 10 ml of methanol to obtain a final concentration. Each stock solution was serially diluted to make different concentrations. Sulfamethazine was diluted to 10, 30, 90, 270, and 810 Tetracycline solution was diluted from stock to 0.5, 1.5, 3.0, 16, and 18 μL, while streptomycin was diluted to 0.5, 1.5, 4.5, 13.5, and 40 Amoxicillin concentration was diluted from stock to 1.5, 4.5, 13.5, 40.5, and 121.5 μL, and erythromycin concentration was diluted to 0.2, 0.6, 1.8, 5.4, and 16.2 μL. The resulting solution was used to test the linearity of the assay.
The HPLC validation was done as follows: five different known concentrations of standards were injected, and the same was injected repeatedly at least three times into the HPLC. The area obtained was recorded, the R2 was calculated, and the retention time and area repeatability were extrapolated. The method was validated, the R2 ranged from 0.9-1, and the retention time was around two minutes.
Data analysis
The statistical analysis of responses from the questionnaire was performed using SPSS software (version 25.0; SPSS Inc. USA), and values of p < 0.05 were considered statistically significant. Data was presented in tables, percentages, and graphs. SPSS software (Independent Samples Test) was used to determine the correlation between ELISA and HPLC results at p < 0.05.
Ethical consideration
Prior to the commencement of the study, ethical approval was obtained from the Animal Health Ethics Committee of the Northwest University (NWU-00734-18-A5). All farmers involved in the study were voluntary participants, and a consent form was issued to each participant before conducting a survey and milk sample collection.
Results
Sociodemographic
All the participants interviewed were Africans (100%), as shown in Figure 1a, and most of them (61%) were males. The majority of the male goat farmers (30%) were between the age of 41-50 years, whilst most females (23%) were between the ages of 31-40 years (Figure 1b). A large number of farmers (71%) had finished grade 12 only with no graduation (Figure 1c), and most of them (32%) had fewer than 20 goats in their flocks. Figure 1d indicates that (86%) of goat farmers were keeping goats with the purpose of selling the meat, while none reported being involved in the goat milk business or the selling of both meat and milk. However, all (100%) farmers confirmed that they milk goats within their herds. Some farmers (62%) used the milk to help feed orphaned kids, cats, and dogs (Figure 1e).
The chi-square test revealed no significant relationship between the age of the participant farmers and the purpose of milking goats, as well as the level of education and number of goats a farmer had (p > 0.05). Figure 1f indicates that all farmers had at least one year of experience in goat farming. The majority (43%) of them had more than three years of goat farming experience, and most (19%) were between 31 to 40 years of age. Figure 1g shows a higher proportion (100%) of antibiotic use in goat farming, and the majority (58%) reported using antibiotics on their livestock only when they were sick or showing signs of infection. When farmers were asked when they had last used drugs on their goats, none reported using any on the day of collection.
Antibiotic use and disease incidence
Incidences where farmers are likely to use antibiotics for their livestock, as considered in this study, are presented in Table I. The use of antibiotics by farmers in this area was mainly linked to respiratory diseases (31%), genitourinary infections (24%), and mastitis (20%). However, no farmer used antibiotics to treat their animals for gastrointestinal tract (GIT) infections. Among the farmers surveyed, 14% reported using erythromycin to treat respiratory diseases, while 13% used sulfamethazine, and 4% reported using amoxicillin. Among the farmers surveyed, 13% reported using tetracycline to treat genitourinary infections, while 3% reported using streptomycin. The chi-square test results indicate that there is no statistically significant association between the drugs used to treat diseases or illnesses (p > 0.05).
When asked where they get the drugs from and how they deal with sick animals, 46% of the farmers indicated that they get their drugs from pharmacies, and 54% reported getting them from either private or state veterinarians (Figure 2). The majority of farmers (33%) choose to consult animal hospitals when their animals fall ill, seeking professional veterinary care. In contrast, a smaller proportion (16%) prefer to self-treat their sick animals. Furthermore, a statistical test with a p-value greater than 0.05 was conducted to explore the potential association between age and the source of antimicrobials among farmers. However, the results did not reach statistical significance. Also, the inferential analysis did not reveal any association between decisions taken in the case of infectious diseases with gender, age, level of education, and degree of farming experience (p-value > 0.05).
In this study, all (100%) participants responded positively when asked about antibiotic guidelines. However, 11% of them were not concerned about the correct dosage, and 4% lacked knowledge about the route of administration. Approximately 95% of the respondents were aware of the withdrawal period, and 65% of them adhered to the withdrawal period (Table II). However, out of the farmers that had knowledge of antibiotic residues, 93% believed they posed health hazards to both the animals and consumers. Moreover, 67% of them were knowledgeable about antibiotic resistance and agreed that it had become a global problem.
Antibiotic residues detection and quantification
ELISA
Out of 266 goat milk samples screened from 13 locations, 265 (99.6%) were positive for one or more antibiotic residue. Only one sample was negative for all antibiotic residues investigated.
The concentration ranges of the antibiotics used in this study are given in Table III. The highest mean concentration was sulfamethazine (369.02 μς/ί), and the lowest was tetracycline (1.9 μς/ί). The occurrence of antibiotics in the samples can be summarised as follows; erythromycin (99.6%), sulfamethazine (84.5%), tetracycline (58.2%), amoxicillin (39.4%) and streptomycin (18.7%). The results also showed that erythromycin, sulfamethazine, and amoxicillin concentrations were above the MRL of Codex Alimentarius in 94.7%, 82.3%, and 35.3% of the samples, respectively. Although tetracycline and streptomycin were detected in the samples, the concentrations were below the MRL of 100 μg/L and 200 μg/L, respectively.
HPLC
The HPLC results confirmed the presence of streptomycin (90%), sulfamethazine (83.3%), erythromycin (53.3%), and tetracycline (40%) in the goat milk samples; however, the established Codex Alimentarius MRL for sulfamethazine (100 μς/ί) and erythromycin (40 μς/ί) were exceeded in 76.6% and 10% of the samples respectively. No amoxicillin residues were detected in any of the raw milk samples (Table IV).
Using the chi-square test, the comparative approach between the results obtained from HPLC and ELISA revealed a significant correlation (p = 0.00) for erythromycin. However, the results were not significant for amoxicillin, tetracycline, sulfamethazine, and streptomycin (p < 0.05).
Discussion
The findings obtained from the present survey revealed that 61% of the respondents were African males, possibly because they are the heads of their families and are responsible for their households, properties, or possessions. The findings in this study are similar to the results obtained by Adebowale et al. (2016), who reported that 71.8% of the farmers interviewed on antibiotic use and practices in commercial poultry-laying hens in Ogun State, Nigeria, were male farmers. Most (43%) of the farmers had more than three years of goat farming experience, and most (19%) were between 31 and 40 years of age.
The results reported in this study show that the majority (30%) of male respondents were mainly in their active age between 41-50 years at the time of data collection, while the female participants were between 31-40 years; André et al. (2010) reported that 146 (19.7%) of the farmers interviewed in a survey were between 41 to 50 years of age. This result indicates that the data were predominantly collected from rural communities with a relatively high proportion of elderly individuals.
The findings also show that 71% of the farmers had attained a grade 12 school education. Most of the farmers had fewer than 20 goats in their flocks. From this may be concluded that your education level does not determine the size of your herd. These findings were similar to the study of Katakweba et al. (2012), who reported that 84% of the farmers interviewed had only ordinary and primary school education.
Most (62%) reported using goat milk to help orphaned kids, cats, and dogs. Only 11% of the farmers reported consuming goat milk. These results correlate with the findings obtained by Zereu et al. (2016), who reported that about 96% (99/103) of the respondents did not consume goat milk in the Humbo District of Wolaita Zone in Southern Ethiopia due to food habits, low amount of milk produced per goat and lack of awareness of the nutritional value of goat milk.
Generally, antibiotics in livestock production are used for both therapeutic and non-therapeutic purposes (Boamah et al. 2016). The existing factors that determine antibiotic usage include: a high prevalence of infectious diseases, poor management and development strategies, a shortfall in husbandry planning, and poor hygiene practices in livestock husbandry associated with an integrated agricultural system (Manyi-Loh et al. 2018). In this study, all the farmers interviewed used antibiotics for their livestock farming, particularly goats, with or without consulting those with the expertise. These results suggested that a veterinarian did not always prescribe antibiotics to treat animals in the sampling area. These practices showed that there could be a possibility of misuse and non-adherence to the guidelines on the use of antibiotics. The high incidence of farmers using antibiotics on their goats is not surprising, especially given that most of the animals in rural areas are exclusively raised on low-quality pastures, frequently infested with parasites, and provided with little preventive measures.
The present study found that 58% of the respondents use antibiotics when the goats are sick or show signs of bacterial infection. These results differ from those obtained by Manishimwe et al. (2017), who reported that 97% of farmers use antibiotics on their animals for disease prevention and growth promotion. The current study reported that 46% of the farmers sourced the antibiotics from pharmacies; Oluwatayo & Oluwatayo (2012) reported that 91.4% of poultry farmers had sourced antibiotics from pharmacy shops, supporting this finding. Horumpende et al. 2018 reported that pharmacies often act as the first point of contact for healthcare. These habits may be explained by the poor policies regarding antibiotic use and poor awareness among farmers about the rational use of antibiotics.
The current study indicates that prevalent diseases in Mahikeng Local Municipality include respiratory diseases (31%), genitourinary (24%), and mastitis (20%). These observations were not concurrent with the report by Parvez et al. 2014, who reported digestive (16.85%), respiratory (9.57%) and urinary system diseases (3.31%) and mastitis (2.02%). The prevalence of skin conditions in this study was found to be 2%, and this result differs from the results obtained by Kabir et al. (2010), who observed 9.56% of skin diseases in goats. The variation in the prevalence of diseases and disorders observed might be due to different geographical locations, number of samples, and study periods.
In the present study, 33% of the participants preferred consulting with an animal hospital, while 26% sometimes consult or self-medicate, and 25% consult with the state veterinarian. About 16% treat sick animals themselves. These results showed that most of the farmers in the study area consult with veterinarians or animal health technicians, as these professionals usually conduct outreach programmes in the study area. The current study showed that many farmers were knowledgeable on antimicrobial residues, antimicrobial resistance, and human health hazards along with correct dosage recommendations, therapy duration, route of administration and withdrawal period. However, the higher proportion of samples containing antimicrobial residues above Codex MRLs indicates that most farmers were not concerned by the withdrawal period or hazards to human health associated with antimicrobial residues.
These results suggest that farmers know about antibiotics and their use. They were aware of the correct dosage, health hazards, and other factors of antibiotic use, but the fact that they did not take these factors into consideration may be due to the lack of legal requirements. These results disagree with the results obtained by Olatoye and Basiru (2013), who reported that a high proportion (75%) of the respondents in their study were not knowledgeable about the deposition and the public health implication of the residues in fish meat.
The results showed that a large proportion of milk produced in the study area contained residues of one or more antibiotic. These results also indicated that consumers of goat milk in Mafikeng Local Municipality are exposed to public health risks associated with the consumption of antimicrobial residues. The inappropriate use of these agents could also accelerate the development of microbial resistance to the different groups of microbes (Boamah et al. 2016).
All the 266 samples collected were subjected to ELISA; the results showed that 50 (18.7%) milk samples had streptomycin residue, and none exceeded the established MRL of 200 μg/L. The study by Bilandžić et al. (2011) on veterinary drug residue determination in raw milk in Croatia showed that the antibiotic neomycin had the highest level in analysed samples, followed by flumequine and streptomycin. The concentration found was lower than the permitted limit proposed by European legislation, corroborating the current results. The potential explanation for samples not exceeding the established MRL in this study could be the non-availability of the streptomycin antibiotic over-the-counter. In veterinary medicine, tetracycline is widely used to treat gastrointestinal, respiratory, and skin bacterial infections, infectious diseases, genitourinary tract, and systemic infections (Granados-Chinchilla & Rodriguez 2017). The extensive use of tetracycline has become a serious problem since it is present as residues in animal products intended for human consumption that can promote the occurrence of antimicrobial-resistant bacteria and its toxicity in humans (Bitas & Samanidou 2018).
Although ELISA detected tetracycline residues in 58.2% of the samples in this study, the concentrations did not exceed the permitted limit of 100 μg/L. This may suggest that most farmers in the study area used the antibiotic tetracycline responsibly, following the right dosage and the withdrawal period. Results from the current study were similar to those of Elizabeta et al. (2011), who detected tetracycline in 50.6% of the milk samples, and none of the positive samples had antibiotic residues above the MRL value.
Amoxicillin is another antibiotic that is used by farmers and was detected in 39.4% of the samples, with 35.3% above the MRLs set by the Codex (Ghidini et al. 2002). Similarly, Khaskheli et al. (2008) analysed 137 milk samples, and 36.5% of the samples were positive for amoxicillin, which corroborates the results obtained in this study.
Notably, 95% of sulfonamide residue violations in animal tissues are due to sulfamethazine (Awaisheh et al. 2019). In this study, sulfamethazine residues were detected in 84.5% of the milk samples, with 82.3% of these positive samples above the MRLs set by the Codex Alimentarius. This high incidence rate and contamination level of sulfamethazine in the current study may indicate that the farmers use this drug extensively. This could also be due to the high effectiveness of the drug in fighting infectious diseases and promoting growth. In contrast, a study by Rama et al. (2017) reported that sulfonamides were detected in 18.4% of analysed milk samples in Macedonia, and the drug residues were below the maximum residue limits.
The incidence of erythromycin in the milk samples was the highest (99.6%), and 94.7% of these positive samples exceeded the permitted limit of 40 μg/L. These results are not surprising as Bilandžić et al. (2011) reported that erythromycin was the most commonly used macrolide. Bansal et al. (2011) also stated that erythromycin is a low-cost macrolide antibiotic and was proven to be very useful for the treatment of infectious diseases. Furthermore, Henton et al. (2011) reported macrolides as the most used antibiotics in livestock.
In a study conducted by Shrivastav et al. (2017) on highly sensitive and selective erythromycin nanosensors employing fibre optic SPR/ERY imprinted nanostructure, no erythromycin residues were detected in the milk samples. This could be due to the different methods used and depends on the farmer's or veterinarian's choice of antibiotics in different locations.
Due to a limited budget and the high cost of the HPLC consumables for each antibiotic, only 30 samples (positives and negatives) were randomly selected for confirmation of results obtained from ELISA.
The HPLC results detected the presence of streptomycin (90%), sulfamethazine (83.3%), erythromycin (53.3%), and tetracycline (40%) in the milk samples collected. The absence of amoxicillin in the confirmatory method in this study could be explained by the fact that ELISA is less sensitive than the HPLC test and lacks specificity (Gaurav et al. 2014). Therefore, there is a possibility that ELISA could have picked up any antibiotic in the same group as amoxicillin leading to false-positive results (Kebede et al. 2014).
In addition, it is known that high buttermilk fat can also obstruct the movement of milk through the assay (immuno-affinity column), causing the chemical reaction to increase, as a result giving an increased probability of false positives (Pereira et al. 2014). Nevertheless, the ELISA method is still used for qualitative antibiotic screening, mainly because of its low cost, simplicity, and fast response. In contrast, HPLC is recognised as an effective technique for the quantitative analysis of antibiotic residues in milk (Reig & Toldrá 2008). The HPLC test has several advantages; it detects insignificant amounts of the residues, presents an extreme sensitivity, and possesses excellent strength of separation, and excellent reproducibility.
This study revealed that tetracycline was detected in small amounts, and the concentration did not exceed the established MRL. The levels were low compared to those obtained in other studies (Abbasi et al. 2011; Al Zuheir 2012). This could probably be due to the small number of animals in the surveyed farms and the different detection methods, or the antibiotic was properly administrated. The tetracycline levels found in this study are similar to those found by Cammilleri et al. (2019).
With regard to the sulfamethazine and erythromycin analyses, the HPLC test showed that about 76.6% of samples analysed exceeded the established MRL for sulfamethazine and only 10% for erythromycin. These results align with those found by Henton et al. (2011), who reported that sulphonamides and macrolides fall under the most frequently used antibiotics in the treatment of livestock. The low percentage of erythromycin residue obtained in the confirmatory test could be explained by the fact that only a few samples were subjected to the test due to the high cost of running the HPLC.
Furthermore, the current study shows a high concentration (90%) of streptomycin residues in goat milk, possibly reflecting the shift in antibiotic classes used by farmers. All the samples analysed for streptomycin were confirmed to be below the MRL (200 μg/L). Similar results were found by Unusan (2009), who studied 60 samples of milk for streptomycin and found the concentrations below the maximum residue limits permitted by Codex Alimentarius. In addition, there was a significant correlation (p < 0.05) between results obtained from ELISA and HPLC for erythromycin residues.
The presence of antibiotic residues in goat milk is not surprising, especially given that most animals were exclusively raised on low-quality pastures, were frequently infested with parasites, and provided with little preventive treatment. Based on the results of this study, milk contaminated with antibiotics above the recommended residue levels is considered unfit for human consumption (Olatoye et al. 2016).
Additional factors may also be responsible for the persistence of drug residue in food of animal origin. The presence of residues may result from failure to comply with the waiting period after the administration of antimicrobials, failure to consult a veterinarian, pharmacist, or animal health technician before using antibiotics, lack of prior training in animal husbandry, illegal or extra-label use of drugs and incorrect dosage, non-existence of restrictive legislation or inadequate enforcement thereof and poor record maintenance of treatment failures (Kozarova & Máté 2000).
The occurrence of antibiotic residues in this study may be attributed firstly to the mismanagement of antibiotics in rural areas, secondly to the overdose of the antibiotic used, and thirdly to a lack of knowledge concerning the drug. It has been reported that several active veterinary drugs are commercially available, and antibiotics are being used more extensively for therapeutic and prophylactic purposes (Manyi-Loh et al. 2018). However, it should be noted that antibiotic residues in milk may have negative implications for consumer health, such as allergic reactions in hypersensitive individuals, thrombocytopaenia, and other more serious toxic effects (Xie et al. 2018).
In addition, control of infectious diseases helps to minimise the use of antibiotics. This reduces the presence of antimicrobial residues in livestock. This strategy requires an emphasis on proper health planning, emphasising preventive management such as good hygiene, properly ventilated buildings, effective biosecurity, animal identification, appropriate vaccination programmes based on evidence-based risk, adequate nutrition, the use of resistant breeds, low stocking rates and access to clean water. Also, administering medication to sick animals in accordance with the instructions on the medication label. Ensure that a full course of antibiotic treatment and the correct dose is followed. Antibiotics should not be used as a preventive measure unless absolutely necessary.
Limitations
The use of convenience sampling may have led to selection bias in favour of farmers who use antibiotics. This likely led to an overestimation of the exact knowledge score. The confirmatory method was expensive; only a few samples were subjected to confirmatory analysis. The absence of amoxicillin in the confirmatory method in this study could be explained by the fact that ELISA is a less sensitive test compared to the HPLC test, and it lacks specificity; however, the samples could have been confirmed using a different confirmatory method, but that was not done due to limited resources. The convenience sampling may have influenced the 100% tetracycline-positive samples in the study, as only lactating animals were sampled.
Conclusion
According to the literature, this is the first study in Mahikeng Local Municipality to evaluate the presence of antimicrobial residues in goat milk and assess the knowledge of goat farmers about the use and practice of antibiotics. The results obtained in the current study indicate a high level of contamination of goat milk with antimicrobial residues, which may have a negative impact on health, thereby reducing the market value. Therefore, there is a need to increase awareness of antibiotic residues in goat milk and its possible public health implications.
Acknowledgment
The authors would like to thank Mr J Ngwane, Prof CM Mnisi, Mr M Koos, and Mr V Mjekula at the Faculty of Natural and Agricultural Sciences at North-West University for their important technical and clinical support.
Conflict of interest
The authors declare they have no conflict of interest directly or indirectly related to the research.
Funding sources
This work was supported by the funds made available by AGRISETA, HWSETA, the Faculty of Natural and Agricultural Sciences, and the Department of Animal Health, North-West University, Mafikeng Campus.
Ethical approval
Prior to the commencement of the study, ethical approval was obtained from the Animal Health Ethics Committee of the North-West University (NWU-00734-18-A5). All farmers involved in the study were voluntary participants, and a consent form was issued to each participant before administering of questionnaire and milk sample collection.
ORCID
KL Ndlovu https://orcid.org/0000-0001-6106-0742
M Mwanza https://orcid.org/0000-0002-9311-6517
N Nleva https://orcid.org/0000-0002-8082-7774
L Ngoma https://orcid.org/0000-0003-2802-4621
References
Abbasi, M.M., Babaei, H., Ansarin, M., et al., 2011, Simultaneous determination of tetracyclines residues in bovine milk samples by solid phase extraction and HPLC-FL method, Advanced Pharmaceutical Bulletin 1(1), 34. [ Links ]
Adebowale, O.O., Adeyemo, O.K., Awoyomi, O., et al., 2016, Antibiotic use and practices in commercial poultry laying hens in Ogun State Nigeria, Revue délevage et de médecine vétérinaire des pays tropicaux 69(1), 41-45. https://doi.org/10.19182/remvt.31170. [ Links ]
Adrian, J., Font, H., Diserens, J.M., et al., 2009, Generation of broad specificity antibodies for sulfonamide antibiotics and development of an enzyme-linked immunosorbent assay (ELISA) for the analysis of milk samples, Journal of Agricultural and Food Chemistry 57(2), 385-394. https://doi.org/10.1021/jf8027655. [ Links ]
Al Zuheir, I.M., 2012, Detection of β-lactams and tetracyclines antimicrobial residues in raw dairy milk for human consumption in Palestine, Walailak Journal of Science and Technology (WJST) 9(3), 277-279. [ Links ]
André, G., Berentsen, P.B.M., Engel, B., et al., 2010, Increasing the revenues from automatic milking by using individual variation in milking characteristics, Journal of Dairy Science 93(3), 942-953. https://doi.org/10.3168/jds.2009-2373. [ Links ]
Andy, Z., 2013, Aminoglycosides in milk using Agilent Bond Elut Plexa SPE, Agilent Poroshell 120, and LC/Tandem MS. [ Links ]
Ashad, H., Waseem, H., Khaliq, R., 2012, Simple and rapid method on high performance liquid chromatography (HPLC) for estimation of streptomycin sulphate, J World Appl Sci 19(5), 645-649. [ Links ]
Awaisheh, S.S., Khalifeh, M.S., Rahahleh, R.J., et al., 2019, Sulfamethazine contamination level and exposure assessment in domestic and imported poultry meats in Jordan, Veterinary World 12(12), 1992. https://doi.org/10.14202/vetworld.2019.1992-1997. [ Links ]
Bansal, B.K., Bajwa, N.S., Randhawa, S.S., et al., 2011, Elimination of erythromycin in milk after intramammary administration in cows with specific mastitis: relation to dose, milking frequency and udder health, Tropical Animal Health and Production 43, 323-329. https://doi.org/10.1007/s11250-010-9692-1. [ Links ].
Belewu, M.A., Adewole, A.M., 2009, Goat milk: A feasible dietary based approach to improve the nutrition of orphan and vulnerable children, Pakistan Journal of Nutrition 8(10), 1711-1714. [ Links ]
Bernacka, H., 2011, Health-promoting properties of goat milk, Medycyna Weterynaryjna 67(8), 507-511. [ Links ]
Bilandžić, N., Kolanović,, B.S., Varenina, I., et al., 2011, Veterinary drug residues determination in raw milk in Croatia, Food Control 22(12), 1941-1948. https://doi.org/10.1016/j.foodcont.2011.05.007. [ Links ]
Bitas, D., Samanidou, V., 2018, Molecularly imprinted polymers as extracting media for the chromatographic determination of antibiotics in milk, Molecules 23(2), 316. https://doi.org/10.3390/molecules23020316. [ Links ]
Boamah, V.E., Agyare, C., Odoi, H., et al., 2016, Practices and factors influencing the use of antibiotics in selected poultry farms in Ghana, Journal of Antimicro 2(2), 120. https://doi.org/10.4172/2472-1212.1000120. [ Links ]
Cammilleri, G., Pulvirenti, A., Vella, A., et al., 2019, Tetracycline residues in bovine muscle and liver samples from Sicily (southern Italy) by LC-MS/MS method: A Six-Year Study, Molecules 24(4), 695. https://doi.org/10.3390/molecules24040695. [ Links ]
Cinquina, A.L., Longo, F., Anastasi, G., et al., 2003, Validation of a high-performance liquid chromatography method for the determination of oxytetracycline, tetracycline, chlortetracycline and doxycycline in bovine milk and muscle, Journal of Chromatography A 987(1-2), 227-233. https://doi.org/10.1016/S0021-9673(02)01446-2. [ Links ]
Clark, S.B., Storey, J.M., Turnipseed, S.B., 2011, Optimization and validation of a multiclass screening and confirmation method for drug residues in milk using high-performance liquid chromatography/tandem mass spectrometry, Journal of AOAC International 94(2), 383-393. https://doi.org/10.1093/jaoac/94.2.383. [ Links ]
De Abreu, L.P., Ortiz, R.A.M., de Castro, S.C. et al., 2003, HPLC determination of amoxicillin comparative bioavailability in healthy volunteers after a single dose administration, J Pharm Sci 6(2), 223-230. [ Links ]
De Villiers, B., Mangold, S., 2002, Chapter 2: The biophysical environment. D. Walmsley J. Walmsley, S. Mangold, M. Kalule-Sabiti, North West Province state of the environment report. Directorate of Environment and Conservation Management, North West Department of Agriculture, Conservation and Environment, South Africa. [ Links ]
Dean, A.G., Sullivan, K.M., Zubieta, J., et al., 2000, Epi Info 2000: a database, and statistics program for public health professionals using Windows® 95, 98, NT, and 2000 computers. [ Links ]
Eagar, H., Swan, G., Van Vuuren, M., 2012, A survey of antimicrobial usage in animals in South Africa with specific reference to food animals, Journal of the South African Veterinary Association 83(1), 1-8. https://doi.org/10.4102/JSAVA.v83i1.16. [ Links ]
Elizabeta, D.S., Zehra, H.M., Biljan, S.D., et al., 2011, Screening of veterinary drug residues in milk from individual farms in Macedonia, Macedonian Veterinary Review 34(1), 5-13. [ Links ]
García-Mayor, M., Paniagua-Gonzalez, G., Soledad-Rodríguez, B., et al., 2015, Occurrence of erythromycin residues in sheep milk, Validation of an analytical method, Food and Chemical Toxicology 78, 26-32. https://doi.org/10.1016/j.fct.2014.12.020. [ Links ]
Gaudin, V., Maris, P., Fuselier, R., et al., 2004, Validation of a microbiological method: the STAR protocol, a five-plate test, for the screening of antibiotic residues in milk, Food Additives and Contaminants 21(5), 422-433. https://doi.org/10.1080/02652030410001667575. [ Links ]
Gaurav, A., Gill, J.P.S., Aulakh, R.S., et al., 2014, ELISA based monitoring and analysis of tetracycline residues in cattle milk in various districts of Punjab, Veterinary World 7(1), 26. https://doi.org/10.14202/vetworld.2014.26-29. [ Links ]
Gavilán, R.E., Nebot, C., Veiga-Gómez, M., et al., 2016, A confirmatory method based on HPLC-MS/MS for the detection and quantification of residue of tetracyclines in nonmedicated feed, Journal of Analytical Methods in Chemistry 2016. https://doi.org/10.1155/2016/1202954. [ Links ]
Ghidini S., Zanardi E., Chizzolini R., et al., 2002, Prevalence of molecules of beta-lactam antibiotics in bovine milk in Lombardy and Emilia-Romagna (Italy), Annali della Facoltadi Medicina Veterinaria-Universitadegli Studi di Parma (Italy) 245-252. [ Links ]
Gonzales, C.A., Usher, K.M., Brooks, A.E., et al., 2009, Determination of sulfonamides in milk using solid-phase extraction and liquid chromatography-tandem mass spectrometry, Agilent Technologies, Pharmaceuticals. Inc. [ Links ]
Granados-Chinchilla, F., Rodriguez, C., 2017, Tetracyclines in food and feedingstuffs: from regulation to analytical methods, bacterial resistance, and environmental and health implications, Journal of Analytical Methods in Chemistry 2017. https://doi.org/10.1155/2017/1315497. [ Links ]
Henton, M.M., Eagar, H.A., Swan, G.E., et al., 2011, Part VI. Antibiotic management and resistance in livestock production, South African Medical Journal 101(8), 583-586. [ Links ]
Hollis, A., Ahmed, Z., 2013, Preserving antibiotics, rationally, New England Journal of Medicine 369(26), 2474-2476. https://doi.org/10.1056/NEJMp1311479. [ Links ]
Horumpende, P.G., Sonda, T.B., van Zwetselaar, M., et al., 2018, Prescription and non-prescription antibiotic dispensing practices in part I and part II pharmacies in Moshi Municipality, Kilimanjaro Region in Tanzania: A simulated clients approach, PloS One 13(11), e0207465. https://doi.org/10.1371/journal.pone.0207465. [ Links ]
Hsieh, M.K., Shyu, C.L., Liao, J.W., et al., 2011, Correlation analysis of heat stability of veterinary antibiotics by structural degradation, changes in antimicrobial activity and genotoxicity, Veterinarni Medicina 56(6), 274-285. https://doi.org/10.17221/1548-VETMED. [ Links ]
Jank, L., Martins, M.T., Arsand, J.B., et al., 2015, High-throughput method for macrolides and lincosamides antibiotics residues analysis in milk and muscle using a simple liquid-liquid extraction technique and liquid chromatography-electrospray-tandem mass spectrometry analysis (LC-MS/MS), Talanta 144 (2015), 686-695. https://doi.org/10.1016/j.talanta.2015.06.078. [ Links ]
Kabir, M.H., Reza, M.A., Razi, K.M.A., et al., 2010, A report on clinical prevalence of diseases and disorders in cattle and goat at the Upazilla Veterinary Hospital, Ulipur, Kurigram, International Journal of Biological Research 2(11), 17-23. [ Links ]
Katakweba, A.A.S., Mtambo, M.M.A., Olsen, J.E. et al., 2012, Awareness of human health risks associated with the use of antibiotics among livestock keepers and factors that contribute to selection of antibiotic resistance bacteria within livestock in Tanzania, Livestock Research for Rural Development 24(10), 170. [ Links ]
Kaya, S.E., Filazi, A., 2010, Determination of antibiotic residues in milk samples, Kafkas Univ Vet Fak Derg 16(Suppl-A), S31-S35. [ Links ]
Kebede, G., Zenebe, T., Disassa, H., et al, 2014, Review on detection of antimicrobial residues in raw bulk milk in dairy farms, Afr J Basic Appl Sci 6(4), 87-97. [ Links ]
Khaskheli, M., Malik, R.S., Arain, M.A., et al., 2008, Detection of ß-lactam antibiotic residues in market milk, Pak J Nutr 7(5), 682-685. https://doi.org/10.3923/pjn.2008.682.685. [ Links ]
Kozarova, I., Máté, D., 2000, Evaluation of the sensitivity of individual test organisms to residual concentrations of selected types of anticoccidial drug, Bulletin of the Veterinary Institute in Pulawy 44(2). [ Links ]
Lee, M.H., Lee, H.J., Ryu, P.D., 2001, Public health risks: Chemical and antibiotic residues-review, Asian-Australasian Journal of Animal Sciences 14(3), 402-413. https://doi.org/10.5713/ajas.2001.402. [ Links ]
Luo, W., Hansen, E.B., Ang, C.Y., Deck, J., et al., 1997, Simultaneous determination of amoxicillin and ampicillin in bovine milk by HPLC with fluorescence detection, Journal of Agricultural and Food Chemistry 45(4), 1264-1268. https://doi.org/10.1021/jf960739l. [ Links ]
Manishimwe, R., Nishimwe, K., Ojok, L., 2017, Assessment of antibiotic use in farm animals in Rwanda, Tropical Animal Health and Production 49 (2017), 1101-1106. https://doi.org/10.1007/s11250-017-1290-z. [ Links ]
Manyi-Loh, C., Mamphweli, S., Meyer, E., Okoh A., 2018, Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications, Molecules 23(4),795. https://doi.org/10.3390/molecules23040795. [ Links ]
Olatoye, I.O., Basiru, A., 2013, Antibiotic usage and oxytetracycline residue in African catfish (Clarias gariepinus) in Ibadan, Nigeria, World Journal of Fish and Marine Sciences 5(3), 302-309. [ Links ]
Olatoye, I.O., Daniel, O.F., Ishola, S.A., 2016, Screening of antibiotics and chemical analysis of penicillin residue in fresh milk and traditional dairy products in Oyo state, Nigeria, Veterinary World 9(9), 948. https://doi.org/10.14202/vetworld.2016.948-954. [ Links ]
Oluwatayo, I.B., Oluwatayo, T.B., 2012, Small ruminants as a source of financial security: a case study of women in rural Southwest Nigeria, Institute for Money, Technology and Financial Inclusion (IMTFI), Working Paper 1, 21. [ Links ]
Parvez, M.A., Faruque, M.R., Sutradhar, B.C., et al., 2014, Clinical diseases and manifestations of goats and cattle recorded at teaching veterinary hospital in Chittagong Veterinary and Animal Sciences University, Bangladesh Journal of Veterinary Medicine 12(1), 73-81. https://doi.org/10.3329/bjvm.v12i1.20467. [ Links ]
Peacock, C., 2005, October. Goats: Unlocking their potential for Africa's farmers. In Proceedings of the Seventh Conference of Ministers Responsible for Animal Resources, Kigali, Rwanda, 31st October-4th November, 23. [ Links ]
Pereira, C., Martins, L.M., Saraiva, L., 2014, LRRK2, but not pathogenic mutants, protects against H2O2 stress depending on mitochondrial function and endocytosis in a yeast model, Biochimica et Biophysica Acta (BBA)-General Subjects 1840(6), 2025-2031. https://doi.org/10.1016Zj.bbagen.2014.02.015. [ Links ]
Rama, A., Lucatello, L., Benetti, C., et al., 2017, Assessment of antibacterial drug residues in milk for consumption in Kosovo, Journal of Food and Drug Analysis 25(3), 525-532. https://doi.org/10.1016/jjfda.2016.07.007. [ Links ]
Ramatla, T., Ngoma, L., Adetunji, M., et al., 2017, Evaluation of antibiotic residues in raw meat using different analytical methods, Antibiotics 6(4), 34. https://doi.org/10.3390/antibiotics6040034. [ Links ]
Reig, M., Toldrá, F., 2008, Veterinary drug residues in meat: Concerns and rapid methods for detection, Meat Science 78(1-2), 60-67. https://doi.org/10.1016/j.meatsci.2007.07.029. [ Links ]
Shrivastav, A.M., Usha, S.P., Gupta, B.D., 2017, Highly sensitive and selective erythromycin nanosensor employing fiber optic SPR/ERY imprinted nanostructure: Application in milk and honey, Biosensors and Bioelectronics 90, 516-524. https://doi.org/10.1016/j.bios.2016.10.041. [ Links ]
Su, S., Zhang, M., Li, B., et al., 2008, HPLC determination of sulfamethazine in milk using surface-imprinted silica synthesized with iniferter technique, Talanta 76(5), 1141-1146. https://doi.org/10.1016/j.talanta.2008.05.015. [ Links ]
Unusan, N., 2009, Occurrence of chloramphenicol, streptomycin and tetracycline residues in ultra-heat-treatment milk marketed in Turkey, International Journal of Food Sciences and Nutrition 60(5), 359-364. https://doi.org/10.1080/09637480701664555. [ Links ]
Xie, Y., Hu, Q., Zhao, M., et al., 2018, Simultaneous determination of erythromycin, tetracycline, and chloramphenicol residue in raw milk by molecularly imprinted polymer mixed with solid-phase extraction, Food Analytical Methods 11(2), 374-381. https://doi.org/10.1007/s12161-017-1008-x. [ Links ]
Zereu G., Meshka M., Shanka M., Sodo E., Agriculture & Healthcare. (2016). Assessment of Goat Production Systems and Factors Affecting Production and Utilization of Goat's milk in Humbo District of Wolaita Zone, Southern Ethiopia, Journal of Biology, Agriculture and Healthcare 6, 5. [ Links ]
 Correspondence:
Correspondence:
email: lubanza.ngoma@nwu.ac.za













