Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.95 n.1 Pretoria 2024
https://doi.org/10.36303/jsava.626
ORIGINAL RESEARCH
Cytological and histopathological bone marrow findings in dogs with natural Babesia rossi infection
MM BumbyI; SJ CliftI; EH HooijbergII; AL LeisewitzII, III
IDepartment of Paraclinical Sciences, Faculty of Veterinary Science, University of Pretoria, South Africa
IIDepartment of Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria, South Africa
IIIDepartment of Clinical Sciences, Auburn University College of Veterinary Medicine, United States of America
ABSTRACT
An inappropriate regenerative response to anaemia has been reported in B. rossi-associated canine babesiosis. We investigated the impact of canine babesiosis on the bone marrow by evaluating the cytological and histopathological changes. Bone marrow smears and histopathology specimens were obtained post-mortem (within 24 hours of death) from six Babesia rossi-infected dogs and five healthy control dogs. Findings were interpreted together with the circulating haemogram, a Perls' Prussian blue special stain for iron and immunohistochemical markers CD3, CD20, MUM-1, MAC387 and CD204. Infected dogs had an inflammatory leukogram, inappropriately regenerative anaemia, hypercellular bone marrow due to erythroid hyperplasia, dyserythropoietic changes within the metarubricyte population, a myeloid hyperplasia with a left shift, a significant increase in the number of CD204-positive resident macrophages, a left shift within the megakaryocyte population and a significantly increased iron content. Whether iron-restricted erythropoiesis plays a role in the anaemia of canine babesiosis could not be established in this study. Our findings concur with what has been recorded in the bone marrow of humans with falciparum malaria and confirm that dyserythropoiesis is partially responsible for the inappropriate erythroid response in canine babesiosis.
Keywords: bone marrow hypercellularity, canine babesiosis, dyserythropoiesis, haemolytic anaemia, macrophage hyperplasia
Introduction
The bone marrow, one of the body's largest organs, is the primary site for haematopoiesis, and is the largest primary lymphoid organ. It also plays a crucial role as a secondary lymphoid organ due to the differentiation of terminal antigen-induced lymphoid cells (Elmore 2006; Travlos 2006). Here we focus on the bone marrow response to canine Babesia rossi infection.
Canine babesiosis is a tick-borne protozoal disease that commonly occurs in South Africa (Collett 2000; Penzhorn 2011). Although various Babesia species infect dogs, Babesia rossi is the most pathogenic of all and is endemic to South Africa (Penzhorn 2011). Clinical signs include fever, anaemia, icterus, tachypnoea, tachycardia, anorexia and splenomegaly (Leisewitz et al. 2019). The anaemia is haemolytic, partially immune-mediated and in some cases, warm in-saline agglutination test (ISA) positive and Coombs'test positive. In addition to haemolytic anaemia, marked acid-base disturbances with secondary multiple organ failure and complications, including acute renal failure, hypoglycaemia, lactic acidosis, hepatopathy with marked icterus, pancreatitis, acute respiratory distress syndrome and cerebral pathology may occur (Leisewitz et al. 2019; Welzl et al. 2001; Jacobson 2006).
From a haematological point of view, B. rossi infection is characterised by an inflammatory leukogram, thrombocytopenia and an inadequately regenerative anaemia (Scheepers et al. 2011). The degree of erythropoiesis/regeneration is best assessed by means of the absolute reticulocyte count (ARC) (Cowgill et al. 2003; Tvedten 2022). Comparing the reticulocyte counts of Babesia-infected dogs with normovolaemic dogs with acute phlebotomy-induced anaemia and a comparable haematocrit, it was found the reticulocyte counts were much lower in Babesia-
infected dogs than in the dogs with phlebotomy-induced anaemia (Scheepers et al. 2011; Spotswood et al. 2005). It was demonstrated that the ARC was < 100 x 109/L in approximately 70% of Babesia-infected cases, which is consistent with a minimally regenerative response to anaemia (Leisewitz et al. 2019). Similar inappropriately regenerative responses were observed during canine babesiosis when compared to dogs suffering from immune-mediated haemolytic anaemia (IMHA) (Seejarim et al. 2023). An inappropriate reticulocytosis relative to the degree of anaemia is also a frequent finding in malaria patients (Weatherall et al. 1983; Kurtzhals et al. 1997). It is possible that, similar to what has been reported in falciparum malaria, this inappropriate regenerative response may be due to a direct effect of the organism on the bone marrow, or that other factors such as inflammation or secondary immune-mediated mechanisms play a role (Scheepers et al. 2011; Seejarim et al. 2023). Dyserythropoiesis may contribute to the poor regenerative response, as erythropoietin levels during canine babesiosis have been found to be sufficient for the degree of anaemia (Onishi et al. 1993). This finding is similar to that found in human malaria (Chang & Stevenson 2004).
Thrombocytopenia is invariably present in B. rossi infections and may be severe (Leisewitz et al. 2019; Kettner et al. 2003). The mechanism of thrombocytopenia is not understood, however, immune-mediated destruction, sequestration of platelets, circulating platelet-leukocyte aggregates and increased consumption (i.e. disseminated intravascular coagulation [DIC]) have been implicated (Goddard et al. 2013; Paim et al. 2012; Goddard et al. 2015a). Thrombocytopenia would normally interfere with maintenance of primary haemostatic function but, despite this, clinical haemorrhage is not observed in the live animal although petechiae, ecchymoses and suffusive haemorrhages are a feature of post-mortem examinations in and on internal organs (Leisewitz et al. 2019; Goddard et al. 2015b; Liebenberg et al. 2013). In malaria, similar to canine babesiosis, thrombocytopenia occurs without a bleeding tendency and appears to be unrelated to DIC (Kueh & Yeo 1982; Wickramasinghe & Abdalla 2000). Thrombocytopenia in human malaria is mostly as a result of a shortened life span of platelets due to either attachment of malarial antigens onto platelets with a subsequent antibody response and platelet phagocytosis, or due to platelet activation and sequestration within the spleen (Wickramasinghe & Abdalla 2000; Skudowitz et al. 1973). A normal number of megakaryocytes are present in the bone marrow and defective thrombopoiesis is not evident (Casals-Pascual et al. 2006; White & Ho 1992).
B. rossi infection has been shown to result in an inflammatory leukogram with the total white cell count being relatively unaffected but with a left shift neutrophilia and lymphopenia (Leisewitz et al. 2019; Rautenbach et al. 2017). The higher the immature neutrophil count and the lower the lymphocyte count, the poorer the outcome (Rautenbach et al. 2017; Leisewitz et al. 2019).
The aim of this study was to describe the histology and immunohistology of the bone marrow of B. rossi-infected dogs which has never been done before. A secondary aim was to compare the poorly regenerative anaemia previously described with B. rossi (Seejarim et al. 2023) infection with the degree of red cell regeneration observed in the bone marrow.
Materials and methods
Experimental design
This study was a cohort (prospective observational) matched case-control study. Six dogs infected with B. rossi, (as diagnosed on a thin peripheral blood smear and PCR-Reverse Line Blot [RLB] hybridisation assays [Matjila et al. 2008]) were included for study. Four of six were euthanised because of poor prognosis and the other two died naturally. This group included dogs of any breed, age, sex and body weight and was independent of whether or not they were treated. This group included four males and two females. Five dogs were naturally infected, and one dog was experimentally infected. All dogs were presented to the hospital for care. The dogs that were euthanised were not admitted for care, whilst the two that died naturally did receive in-hospital care. Only one of these dogs had severe anaemia alone. The others all had a combination of body systems showing evidence of dysfunction and/or failure (acute respiratory distress syndrome, hepatosis and icterus, pancreatitis, coagulopathy, acute kidney injury, profound intravascular haemolysis). The details of the experimental infection are described elsewhere (van Zyl et al. 2022; Atkinson et al. 2022; Smith et al. 2021). Infected animals were excluded from the study if routine haematology (complete blood count [CBC] including manual differential count) were not performed prior to death, if they were positive for parasites other than B. rossi (by PCR-RLB assay), or had concurrent (non-Babesia related) severe disease determined on post-mortem examination (such as neoplasia, severe helminthiasis, or inflammatory disease). One infected dog did not have a reticulocyte count performed. Five control dogs were obtained from a welfare organisation after humane euthanasia. These dogs ranged in age from one to four years old, were all medium-sized mixed breed and were all intact females. Data was collected with the approval of the Animal Ethics Committee of the University of Pretoria, South Africa (protocol V034-14 and V047-18). Control dogs were deemed healthy based on the clinical examination, CBC, negative PCR-RLB hybridisation results (on blood) and absence of significant lesions or abnormalities at post-mortem.
Blood sample collection
Blood samples for a full haematology assessment were collected from all study and control dogs within three hours before death. Samples consisted of 5 mL of peripheral venous blood collected via jugular venipuncture with a 21-gauge needle and placed into an EDTA vacutainer tube (BD Vacutainer K2E [EDTA] 7,2 mg). A 1 mL aliquot of this EDTA anticoagulated blood was stored at -80 °C for subsequent PCR-RLB assays to confirm B. rossi monoinfection as previously described (Matjila et al. 2008).
Haematology
An automated CBC was performed on the blood samples (ADVIA 2120i, Siemens Healthcare, South Africa). A 100-cell differential white blood cell count was conducted manually for each dog by a trained veterinary haematology technician and the differential count (expressed as 109 cells/L) was recorded. A separate automated reticulocyte count was performed for five of the six infected dogs and all of the control dogs, and the ARC was established (109 reticulocytes/L). Various reticulocyte indices as generated by the ADVIA 2120i were also captured and used in the assessment of the anaemia.
Cytology and histopathology specimen collection
Post-mortem examinations were performed at the Section of Pathology, Department of Paraclinical Sciences, Faculty of Veterinary Science, University of Pretoria, by the principal investigator. To minimise pre-analytic error, the collection and preparation of bone marrow samples was standardised for both study and control cases. The interim was recorded for each case. Bone marrow cytology and histopathology samples were obtained within two hours after death to minimise autolytic change. Bone marrow samples were collected from the metaphysis of both (left and right) humeri and femori to ensure adequate sample size (approximately 1 cm3) for both cytology and histopathology. The proximal metaphysis of the humeri and femori were opened using secateurs in order to avoid heat damage to the cells associated with sawing bones. Samples for bone marrow cytology were collected by gently breaking off pieces of bony trabeculae using an 18-gauge needle and lightly rolling the bony spicules over a clean microscope glass slide, taking care not to roll the material more than two-thirds of the way down the slide. For cytological evaluation, 10 slides were prepared per patient. Smears were initially stained with Wright Giemsa (Siemens Hematek 2000 autostainer). Initial staining was not considered adequate due to the thickness of the smears, and slides were further hand-stained with a modified Romanowsky stain (Hema-Quik, Thermo Fisher Scientific, South Africa) until optimal staining intensity was achieved.
For histopathological evaluation, at least four 5 mm x 5 mm samples of metaphyseal bone marrow (one per humerus and one per femur) were immersed in 10% neutral buffered formalin. This included the cortical bone of the longitudinally sectioned proximal humeri and femori to avoid the dispersion of the bone marrow sample. Samples were fixed for 48 hours after which the samples were wax-embedded, and routinely stained with haematoxylin and eosin (HE).
Histopathology and cytology
Bone marrow cellularity was histologically evaluated over three high power fields/HPF (x 400 magnification) per slide and scored on a scale of 1-4. A score of 1 represented 0-25% cellularity, score 2 represented 26-50% cellularity, score 3 represented 51-75% cellularity and score 4 represented 76-100% cellularity. Megakaryocytes were evaluated over 3 HPF and numbers were scored as 1/low (< 2/200 x magnification), 2/normal range (2-3/200 x magnification) or 3/high (> 3/200 x magnification) (Lucidi et al. 2017). Endothelial cell hypertrophy was evaluated by comparing endothelial cell nuclear diameter to erythrocyte diameter. The score was designated as 0 when the endothelial cell nuclear diameter was less than or equal to that of a red blood cell, and 1, when the endothelial cell nuclear diameter was greater that the diameter of a red blood cell. Babesia parasites were counted in the bone marrow histology sections to investigate their density and potential sequestration, despite the lower sensitivity compared to peripheral blood smear counts. Parasitised red blood cells (pRBCs) were evaluated over three capillary segments and scored: 0 for < 50% pRBCs and 1 for > 50% pRBCs. The number of pRBC was evaluated over three capillary segments. The pRBC were evaluated in relation to non-pRBC and a score of 0 designated less than 50% pRBC and a score of 1 designated more than 50% pRBC.
The cytological evaluation was performed in a blinded manner by a European board-certified clinical pathologist (EH), using a systematic approach (Haddad & Grindem 2020). Cellularity was estimated after examining all slides for a dog. The average number of megakaryocytes per 10 x objective field was calculated from 10 fields. A 500-differential cell count was performed and the following calculated: myeloid:erythroid (M:E) ratio, the number of the different myeloid and erythroid precursor cells as a percentage of all nucleated cells, and as a percentage of all myeloid or erythroid cells. The percentage of lymphocytes, plasma cells and histiocytes/macrophages, compared to all nucleated cells, was also calculated. The presence or absence of iron stores, stromal reaction and Babesia parasites was also noted. Morphological abnormalities in any cell lines were also noted. Final interpretation of bone marrow findings took into account the results of the concurrent CBC results.
Immunohistochemistry and special stains
Manual indirect chromogen-based immunoperoxidase staining was performed according to standard procedures. Immunohistochemical markers included CD3 for T-lymphocytes (Dako Catalogue number: A0452), CD20 for B-lymphocytes (Dako Catalogue number: Thermo scientific - PA5-16701), MUM1 for mature B-lymphocytes and plasma cells (Dako Catalogue number: M7259), MAC387 for monocytes/macrophages and mature granulocytes within the bone marrow (Dako Catalogue number: M0747) and CD204 for resident/tissue macrophages with variable expression in dendritic cells (Abnova Catalogue number: MAB1710). Perls' Prussian blue for ferric iron was applied to the sections. Virtual slides were generated for the IHC-treated slides using an Olympus VS120-S6-W whole slide digital scanner (Wirsam Scientific). Olympus cellSens Dimension software (Olympus cellSens V, Olympus, Japan) was used to count and measure regions of interest (ROI) in the IHC-treated slides. Per slide, four ROI were determined, each incorporating the largest possible tissue area without the presence of artefacts such as slits, spaces, uneven staining and tissue folds. The mean percentage of positively labelling cells was determined per slide.
Statistical analysis
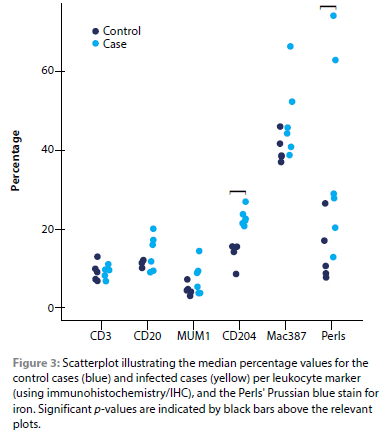
The study was descriptive at the haematological, cytological, microscopic and immunohistochemical level and as such did not rely heavily on statistical testing, however, non-parametric tests were conducted to compare medians and to determine correlations. Statistical analysis was performed by using a statistical software package (SPSS, version 24, IBM). The significance between the medians of the sample groups (control and infected) for the CBC variables, cytology differential cell counts, immunohistochemical and special stain percentages, were compared by performing a Mann-Whitney U test. Spearman's rho was used to determine correlations. Significance was set at p < 0.05. Figure 3 was generated by means of R software for statistical computing (https://www-r-project.org).
Results
Haemogram
Erythron, reticulocyte and leukon variables are summarised in Tables I to III. All infected cases were anaemic (median haematocrit 0.11 L/L, range 0.06-0.19 L/L, p = 0.004). This anaemia was deemed severe in one of the infected cases (defined as a haematocrit of 0.13-0.19 L/L) and very severe within the remaining five cases (defined as a haematocrit of < 0.13 L/L) (Tvedten 2022). In the five infected cases where reticulocytes were measured, this anaemia was poorly regenerative based on the ARC (median 66.6, range 31.6-132.0 X 109/L) (Tvedten 2022). In addition to significantly lower haemoglobin, haematocrit, red cell concentrations and reticulocyte cell haemoglobin concentration mean (CHCMr) in the Babesia-infected cases compared to the control group, significantly increased values were observed for the following variables (Tables I and II): red cell distribution width (RDW), nucleated red blood cells (i.e. erythrocyte precursors) per 100 white blood cells (NRBC/100WBC), reticulocyte percentage (RET%) and the distribution width (variability) of CHr (CHDWr). In all of the infected cases, regeneration was weak based on the ARC (i.e. ARC values of < 150.0 x109/L) (Tvedten 2022). The reticulocyte haemoglobin content (CHr) was decreased below the reference interval (Schaefer & Stokol 2016) in one of the infected cases whereas the remaining CHr values for the Babesia cases were within the reference interval. Five of the six infected cases had a leukocytosis with a left shifted neutrophilia, whilst the experimentally infected case had a leukopenia with neutropenia and a left shift. A thrombocytopenia varying from mild to severe was noted in five of the six infected cases. A warm ISA test was negative in all dogs.
Cytology
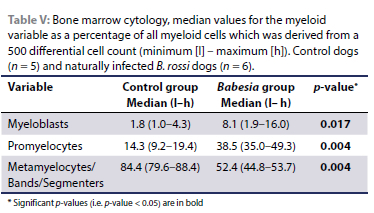
Bone marrow cellularity, in particular erythroid cellularity, was increased in all infected cases, with the myeloid:erythroid ratio (M:E) ranging from 1:1.2 to 1:4.0 (M:E controls 1:0.4-1:1.3). Of the erythroid precursors, only the rubriblasts were significantly higher in the Babesia-infected dogs than the controls (Table IV). Orderly maturation and unremarkable morphology of the erythroid lineage were noted within the control group (Figure 1A), whereas an erythroid left shift was observed in four of the six infected cases. Dysplastic changes, including nuclear pleomorphism and binucleation, were observed within metarubricytes of all infected animals, varying from mild to moderate (Figure 1B). Compared to the normal myeloid cellularity, orderly maturation and unremarkable morphology in the control group samples, increased myeloid cellularity was observed in all infected cases (Figure 1B; Table V). The myeloid lineage in these cases was characterised by a moderate to severe left shift, with the maturation pool comprising mostly metamyelocytes and bands (Figure 1B), with a marked decrease in segmented forms (Figures 1B and C). Apart from the normal morphology observed in one of the infected cases, metamyelocytes/bands/ segmenters were often giant, with some toxic changes visible, including cytoplasmic basophilia and granulation (Figure 1B). In five of the six infected cases, there was an increase in the number of eosinophils, varying from mild to marked. A low number of mature plasma cells (< 2% of all nucleated cells) was observed in all control cases, whereas the plasma cell percentage was variable in the infected cases, where three cases had > 2% plasma cells (2.4%, 6.6%, 19.4%). In four out of the six infected cases, immature plasma cells were noted. Lymphocytes were mostly small with increased medium-sized lymphocytes noted in three of the cases. Large granular lymphocytes were seen in two infected cases. Macrophages and histiocytes were increased and comprised more than 10% of the total nucleated cell count in four Babesia cases (Figures 1C and D). Macrophages were moderately to very active in five out of the six Babesia cases. Where active, approximately 50% of the macrophage population showed vacuolisation, erythrophagocytosis and the accumulation of intracytoplasmic haemosiderin pigment (Figure 1D). Two Babesia cases were associated with low numbers of macrophages (< 5%), mirroring observations in the control dogs. Megakaryocyte numbers were variably increased in four of the infected cases. This was associated with a variable left shift towards immaturity in megakaryocytes. In two of the Babesia cases as well as all the control cases, the number of megakaryocytes were within normal limits.
Histopathology and immunohistochemistry
In all the Babesia-infected cases, the bone marrow was markedly hypercellular/hyperplastic (Figures 2A and B). There was also a consistent increase in the number of megakaryocytes, with a predominance of immature forms (Figure 2B). Endothelial cell hypertrophy/activation was observed in two of the six Babesia-infected cases, which coincided with the presence of moderate diffuse congestion and multifocal haemorrhages. Thrombosis was not observed in any of the cases. One of the infected cases, which was untreated, had > 50% pRBCs, while the remaining five infected cases exhibited < 50% pRBCs. Erythrophagocytosis was prominent in all the infected cases. A significant increase in CD204-immunoreactive macrophages and Perls' Prussian blue-positive staining of iron deposits was observed in the infected dogs compared to the controls (Figures 2C-F and Figure 3; Table IV). No significant differences were detected in T-lymphocytes (CD3), B-lymphocytes (CD20), plasma cells (MUM1), or MAC387-reactive granulocytes and monocyte-macrophages between the control and infected groups (Figure 3).
Discussion
We have shown an inappropriate bone marrow response to the haemolytic anaemia and systemic inflammation caused by B. rossi infection in dogs. The mean RET% indicates the percentage of reticulocytes within a given blood sample (Stockham & Scott 2008), and the significantly increased RET% in the infected group is consistent with Babesia-induced reticulocytosis. The adequacy of the response is, however, seen in the ARC and not the RET%. Regenerative anaemia is characterised by an ARC that is above the upper reference limit (internal laboratory upper reference limit of 115.0 x 109/L here) but is further characterised as weak if the ARC is < 150.0 x 109/L (Tvedten 2022). In this study, the median ARC values were not increased compared to the control group, consistent with an inappropriate marrow response to the anaemia in the Babesia cases (Tvedten 2022). It has been demonstrated intraerythrocytic parasites (such as Babesia and Plasmodium species) can result in a falsely elevated ARC as the ADVIA dye used to detect reticulocytes will also label intraerythrocytic parasites (Sharma et al. 2022; Piane et al. 2016; Laurencet et al. 1997). The parasitaemia of the dogs in this study did not exceed approximately 5% and hence any effect would have been small (de Villiers et al. 2020). It should also be noted that this effect would falsely elevate the ARC thus falsely skew the data towards a more regenerative red cell response. Despite this, the anaemia observed in these cases was still inappropriately regenerative. Despite the effect that this artifact may have introduced to the ARC finding, we do not believe that it alters our findings in any significant way. This finding of a minimally regenerative response to the anaemia is similar to that demonstrated in previous studies (Spotswood et al. 2005; Scheepers et al. 2011; Leisewitz et al. 2019; Seejarim et al. 2023). A retarded reticulocyte maturation time has also been demonstrated during B. gibsoni infection in vitro (Hossain et al. 2007). The authors of this work postulated that the parasite itself and/or various metabolites that are released may be the cause for this delay in reticulocyte maturation (Hossain et al. 2007).
Haemolytic anaemia is expected to induce a proliferation of erythroid precursors (rubriblasts) in what is termed a "left shift" (a cell population shift towards immaturity). The normal pyramidal architecture of maturation of bone marrow cells (most cells being mature and the smallest population being immature) is disrupted as early progenitor cells increase and late-stage mature cell numbers decrease. The median percentage of metarubricytes was decreased in the B. rossi-infected group compared to the control group, although this difference did not attain statistical significance. This may suggest that there is suppression of late-stage erythropoiesis leading to inadequate production and release of reticulocytes - in other words, ineffective erythropoiesis. In addition to this, dysplasia was observed in the metarubricyte population. These effects may be mediated by direct (e.g. parasite-derived soluble factors) and/ or indirect (e.g. secondary to the host inflammatory response) effects on the bone marrow. One could postulate that an element of precursor-targeted immune-mediated destruction may be occurring, possibly secondary to the infection or cytokine imbalance, despite the absence of obvious rubriphagocytosis and collagen fibrosis. It has been shown that the ARC in dogs with IMHA is significantly higher than in Babesia-infected dogs (Seejarim et al. 2023). Interestingly, it has been shown that the dog peripheral blood cytokine response to IMHA, sepsis and B. rossi infection is similar, which suggests that a parasite-mediated effect on the bone marrow in babesiosis may be key to the poor response seen in babesiosis (Leisewitz et al. 2019; Goddard et al. 2016). Similar findings are evident in malaria, where selected cytokines (interferon gamma [IFN-y], TNF-α, IL-2, IL-12 and IL-10) inhibit erythroid progenitor cells or blunt their response to erythropoietin, thereby suppressing erythropoiesis (Wickramasinghe & Abdalla 2000).
Our study demonstrated marrow erythroid hyperplasia, which was not translated into the expected strongly regenerative response expected in the circulating blood. Bone marrow erythroid hyperplasia can be broadly divided into disorders associated with effective erythropoiesis and those associated with ineffective erythropoiesis (Haddad & Grindem 2020). Ineffective erythropoiesis is characterised by erythroid hyperplasia occurring together with a nonregenerative or weakly regenerative anaemia (Stockham & Scott 2008), as was evident in this study. These two contrasting findings have been described in immune-mediated destruction of nRBC, and in various nutritional deficiencies, including iron-deficiency anaemia (Stockham & Scott 2008). It should be borne in mind, however, that a full regenerative response takes approximately four to eight days after the onset of anaemia, when the reticulocyte peak is reached (Tvedten 2022). Interpreting the reticulocyte response early in the course of anaemia may therefore be deceiving as simply not enough time has passed to generate sufficient numbers of reticulocytes. Similarly, interpretation of the reticulocyte response late in the course of anaemia, i.e. 10-14 days, may give a false impression of poor regeneration since reticulocyte numbers will be decreasing during this stage. In this study, the duration of illness was unknown for one of the infected dogs, with a three-day median duration of sickness in the remaining infected dogs (range three days to 14 days) making the poor reticulocyte response unlikely to be because of the timing of the sample collection. It is even less likely to be as a result of a poorly timed sample collection if one considers the other babesiosis studies that have shown a similarly poorly regenerative response in what is collectively hundreds of cases (Scheepers et al. 2011; Leisewitz et al. 2019).
The significantly increased number of nRBC in the peripheral circulation (specifically more than 0.5 nRBC/100 WBC) in the Babesia-infected group indicates rubricytosis (Dank et al. 2020). Rubricytosis, amongst other things, indicates regenerative anaemia, but its presence also reflects splenic pathology, likely associated with an altered splenic microenvironment, also a feature of Babesia infection (Henning et al. 2020; Mandell et al. 1989). Inappropriate rubricytosis (when seen in the face of a poorly regenerative anaemia) may occur during extramedullary haematopoiesis (EMH), (notably splenic). The spleen in Babesia-infected dogs is characterised by EMH (Henning et al. 2020). The significantly altered splenic architecture that is seen during Babesia infection may also contribute to the rubricytosis. Additionally, despite the lack of obvious histological evidence of bone marrow injury, alteration in the marrow microenvironment could be a contributing factor.
Dyserythropoiesis, which refers to a variety of disorders where erythrocytes mature abnormally or have dysplastic features, can contribute to a poorly regenerative anaemia (Harvey 2012; Parmar & Sheikh 2015). Erythroid dysplasia was observed in all the infected cases in this study. It was characterised by nuclear pleomorphism and binucleation in metarubricytes. Dyserythropoiesis, characterised by erythroid dysplasia, has been reported in various parasite-induced anaemias, including falciparum malaria and leishmaniasis, where it is related to TNF-α and IL-12 production (Wickramasinghe & Abdalla 2000; Abdalla et al. 1980; Wickramasinghe et al. 1989; Mohan & Stevenson 1998; Fleming 1989; Knuttgen 1987; Abdalla 1990; Sheikha 2004). In human malaria, the host immune response to infection, as well as the presence of parasite-derived products are speculated to be the cause of dyserythropoiesis (Pathak & Ghosh 2016).
Systemic inflammatory disease may cause selective erythroid hypoplasia and ineffective erythropoiesis without generalised marrow hypoplasia and may well contribute to the anaemia seen in our study (Stockham & Scott 2008). The pathogenesis involves a reduced erythrocyte lifespan, impaired iron mobilisation or utilisation and impaired production of erythrocytes (Stockham & Scott 2008). The anaemia of inflammation is not associated with an iron deficiency but rather with a maldistribution and unavailability of iron stores (Weiss et al. 2019). Erythrocyte production may be impaired due to the unresponsiveness of erythroid cells to increased EPO levels. This is due to the inhibitory effects of inflammatory cytokines such as IL-1, IFN and TNF on erythroid precursors (Stockham & Scott 2008). Babesia rossi infection has been associated with an excessive pro-inflammatory cytokine response and is a classical example of a systemic inflammation (Goddard et al. 2016; Leisewitz et al. 2019). Similar findings are evident in malaria, where cytokines (IFN-y, TNF-α, IL-2, IL-12 and IL-10) inhibit erythroid progenitor cells or blunt their response to EPO, thereby suppressing erythropoiesis (Wickramasinghe & Abdalla 2000). In a murine model of malaria it was found that erythropoietic cytokines (such as Granulocyte-Colony Stimulating Factor, Granulocyte-Macrophage Colony Stimulating Factor, IL-7, and IL-17) were under expressed whilst regulatory cytokines (such as IL-10 and TNF-α) were over expressed. This demonstrated a markedly dysregulated cytokine network (distinct from what was seen with blood-loss anaemia or non-parasite-induced haemolysis) that had obvious negative effects on erythropoiesis (Xu et al. 2013).
Iron deficiency is another possible cause of a poorly regenerative anaemia. Reticulocyte indices (including mean cell volume of reticulocytes [MCVr], mean haemoglobin concentration of reticulocytes [CHCMr] and haemoglobin content of reticulocytes [CHr]) are used to evaluate the etiopathogenesis of anaemia (Seejarim et al. 2023) and iron-restricted erythropoiesis is indicated by a reduction in these indices (Schaefer & Stokol 2016). The lack of a significant decrease in the MCVr and CHr indices in the infected dogs in this study (despite a significant decrease in the CHCMr values), meant that we were unable to establish that iron-restricted erythropoiesis plays a role in the anaemia of canine babesiosis. A Perls' Prussian blue stain showed a significantly increased amount of ferric iron in the bone marrow of the infected group. A recent publication established the usefulness of grading iron stores in canine bone marrows to establish if an anaemia is due to iron deficiency or not (Pawsat et al. 2023). Although this method was not used in the current study, the lack of significant decreases in MCVr and CHr (despite a significant decrease in CHCMr) and increased bone marrow ferric iron suggests sufficient iron stores. However, the availability of iron for erythropoiesis is uncertain due to possible iron sequestration in hepatocytes, enterocytes and macrophages by hepcidin and inflammatory mediators (Pawsat et al. 2023). The acute nature of B. rossi infection may prevent the anaemia from persisting long enough for iron sequestration to result in iron deficiency anaemia in reticulocyte indices. Thus, iron stores appeared to be sufficient during infection, although whether this iron is available for red cell production is uncertain. It is possible that because the anaemia of babesiosis is acute, enough time would not have passed for the abundant but possibly unavailable iron stores in the marrow to reflect in the various reticulocyte indices as an iron deficiency anaemia.
This study demonstrated an overall increased number of leucocytes in the bone marrow and peripheral circulation of the infected group. There were significantly increased numbers of band neutrophils in the peripheral circulation, a significantly increased number of myeloblasts and promyelocytes in the bone marrow smears, and a significantly increased number of CD204-reactive macrophages in histologic specimens of bone marrow. Marrow cytology showed increased medium-sized lymphocytes and plasma cells in 50% of infected cases, but immunohistology revealed no significant increase compared to controls. The negligible difference in T-cell populations between infected and control groups aligns with findings in the canine spleen during babesiosis (Henning et al. 2020). Hypotheses include a decline in T-lymphocytes in complicated Babesia cases due to mechanisms like accelerated apoptosis, rapid redistribution of effector T-cells (Rautenbach et al. 2017), and suppression by "anti-inflammatory"/alternatively activated macrophages (Henning et al. 2020). The CD204 scavenger receptor A protein, expressed on significantly increased marrow macrophages in infected dogs in this study, may indicate the presence of "anti-inflammatory" macrophages (Nolte et al. 2017; Seung et al. 2018), although further investigation is needed (Belluco 2018). These macrophages regulate immunity, aid tissue repair, and suppress T-cell function in infections and malignant neoplasia (Chow et al. 2022; Seung et al. 2018).
This study found no significant increase in B-cells and plasma cells in infected animals. Disruptions in B-cell lymphopoiesis (Bockstal et al. 2011) and memory B-cell maintenance (Weiss et al. 2009) have been noted in mouse and human malaria studies, with resistance to the early stages of malaria and babesiosis being antibody-independent (Brown 1999; Grun & Weidanz 1981) but antibodies are required for parasite clearance post-acute infection (Fell & Smith 1998). Insufficient data limits the exploration of parasite-induced suppression of B-cell lymphopoiesis and memory B-cell production in the current study.
The observed myeloid left shift and macrophagic response in the bone marrow are consistent with the systemic inflammation caused by Babesia (Martin et al. 2023). Although both CD204 and MAC387 reactive mononuclear leukocytes were elevated in the bone marrow of infected dogs, only CD204 reactive macrophages showed a significant increase. The lack of significant MAC387-reactive cells in the bone marrow was unexpected, given the significantly increased numbers of these cells and CD204-reactive macrophages in the lungs (Martin et al. 2023) and spleen (Henning et al. 2020) of dogs with fatal B. rossi infection. The potential terminal mobilisation of the MAC387-reactive monocyte-macrophages, distinct from CD204-reactive tissue-resident macrophages, from the bone marrow in the final stages of disease could account for their relatively lower numbers in the bone marrow compared to other infiltrated organs.
CD204 reactive macrophages were significantly increased in the marrow of Babesia-infected dogs. This finding is similar to previous IHC studies investigating the effects of canine babesiosis on the lung and spleen (Martin et al. 2023; Henning et al. 2020). An increase in macrophages within the bone marrow has also been demonstrated in human falciparum malaria (Wickramasinghe & Abdalla 2000; Abdalla 1990). Macrophages play a pivotal role in innate immunity where they are crucial for parasite phagocytosis as well as the release of cytokines, chemokines and toxic mediators, and their increased numbers in the infected group in this study was therefore not surprising (Corbett et al. 2021). In addition, increased levels of MCP-1 (a macrophage-derived cytokine) are a feature of B. rossi infection in dogs (Goddard et al. 2016; Leisewitz et al. 2019; Atkinson et al. 2022; van Zyl et al. 2022). It seems clear from this study and the other work cited that the tissue macrophage is an important player in Babesia pathogenesis (probably driving an innate immune response) and is likely to be one of the drivers of the bone marrow pathology we have described.
The myeloid hyperplasia and left shift seen in the infected dogs were to be expected and were consistent with the acute systemic inflammation. A left shift neutrophilia has previously been associated with disease severity and prognosis and is reflective of the very inflammatory nature of the disease (Leisewitz et al. 2019). Much of the pathology is likely host rather than parasite driven resulting in significant tissue damage with the demand for neutrophils outstripping the bone marrow's capacity to provide, leading to the left shift. Neutrophilic metamyelocytes, bands and segmented neutrophils in the infected group were often giant with some toxic changes evident (cytoplasmic basophilia and granulation), indicative of the severity of the inflammation.
In the bone marrow of infected dogs, megakaryocyte numbers were adequate to increased with evidence of a left shift. This is an appropriate response in light of the peripheral thrombocytopenia, which is a common finding in canine babesiosis (Kettner et al. 2003). The pathogenesis of thrombocytopenia in canine babesiosis is not fully understood, although, from the limited data in this study, bone marrow suppression with decreased platelet production appears unlikely. Other studies with similar findings concluded that the thrombocytopenia associated with canine babesiosis is likely due to immune-mediated destruction (Scheepers et al. 2011), sequestration of platelets in circulating platelet-leukocyte aggregates (Goddard et al. 2015a) and/ or increased consumption (Kirtz et al. 2012), such as with DIC, which has been shown in severe forms of the disease (Goddard et al. 2013).
There was some evidence of endothelial reactivity in our cases. Babesiosis is a red cell infection that can result in significant organ damage and the interface between circulating blood and organ tissues is the endothelium. Reactivity of this crucial layer of cells lining the vasculature has been demonstrated to be instrumental in bacterial sepsis (Ince et al. 2016), malaria (Alencar Filho et al. 2014) and canine babesiosis (Baric Rafaj et al. 2013) and is a layer of cells deserving of further investigation in canine babesiosis pathogenesis.
The limitations of this study include the small number of cases evaluated and an incomplete evaluation of the iron status of the dogs. Furthermore, the study was conducted on dogs that died and provided a picture of a snapshot in time only. How the changes evolve over time and if changes in survivors differ from what is seen in those that die, remains unknown. A number of the dogs had been treated with a highly effective babesiocide and this would have prevented an accurate estimation of bone marrow parasite density.
Conclusions
Fatal Babesia rossi infection in dogs resulted in severe extravascular haemolysis with a poorly regenerative anaemia, as indicated by low ARC. Bone marrow pathology was characterised by significant hypercellularity. There appeared to be abundant iron stores in the bone marrow specimens suggesting that absolute iron deficiency does not play a role in the anaemia. There was also evidence of dyserythropoiesis. The marrow erythroid response in infected dogs mirrored findings from previous studies on canine babesiosis and human malaria (Scheepers et al. 2011; Leisewitz et al. 2019; Seejarim et al. 2023). The inappropriate response is likely due to a variety of parasite and host factors. Examples of host factors include pro-inflammatory cytokine-induced reduced erythrocyte survival, impaired iron mobilisation or utilisation, impaired erythrocyte production due to nonresponsiveness to EPO, and dyserythropoiesis. The left shift neutrophilia and very inflammatory nature of the disease seen in the peripheral blood was reflected in the bone marrow. The prominent macrophage response in the marrow is a consistent finding in tissues from Babesia rossi-infected dogs, as it has been reported in the spleen, lungs, brain and liver. The thrombocytopenia that is consistently observed in canine babesiosis probably originates peripherally due to immune-mediated destruction, sequestration or increased consumption of platelets, as megakaryocytes were adequate or increased in number in the marrow in the current study.
Acknowledgements
We thank Dewald Noeth at Wirsam Scientific for assistance with the Olympus CellSens Dimension software program, Eric Liebenberg at the University of the Witwatersrand, Faculty of Health Sciences, for the generation of whole slide digital images, as well as the technologists in the histopathology and clinical pathology laboratories at the Faculty of Veterinary Science, University of Pretoria, for slide preparations.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding source
This work was supported by funding provided by the National Research Foundation (South Africa); Grant number CPRR13080726333. The funding agency played no role in the research project or preparation of the manuscript for publication.
Ethical approval
Ethical approval was obtained from the Animal Ethics Committee of the University of Pretoria, South Africa (protocol V034-14 and V047-18).
ORCID
MM Bumby https://orcid.org/0000-0003-0168-5956
SJ Clift https://orcid.org/0000-0003-1368-1215
EH Hooijberg https://orcid.org/0000-0002-4367-799X
AL Leisewitz https://orcid.org/0000-0001-8432-9425
References
Abdalla, S.,1990, Hematopoiesis in human malaria, Blood cells 16, 401-16; discussion 417. [ Links ]
Abdalla, S., Weatherall, D.J., Wickramasinghe, S.N., et al., 1980, The anaemia of P. falciparum malaria, Br J Haematol 46, 171-183. https://doi.org/10.1111/j.1365-2141.1980.tb05956.x. [ Links ]
Alencar Filho, A.C., Lacerda, M.V., Okoshi, K., et al., 2014, Malaria and vascular endothelium, Arq Bras Cardiol 103, 165-9. https://doi.org/10.5935/abc.20140088. [ Links ]
Atkinson, B.K., Thompson, P., Van Zyl, E., et al., 2022, Kinetics of the inflammatory response during experimental Babesia rossi infection of beagle dogs, Veterinary Parasitology 109717. https://doi.org/10.1016/j.vetpar.2022.109717. [ Links ]
Barić Rafaj, R., Kuleš, J., Selanec, J., et al, 2013, Markers of coagulation activation, endothelial stimulation, and inflammation in dogs with babesiosis, Journal of Veterinary Internal Medicine 27, 1172-1178. https://doi.org/10.1111/jvim.12146. [ Links ]
Belluco, S., 2018, Letter to the Editor, Vet Pathol 55, 597. https://doi.org/10.1177/0300985818763717. [ Links ]
Bockstal, V., Geurts, N., Magez, S., 2011, Acute disruption of bone marrow B lymphopoiesis and apoptosis of transitional and marginal zone B cells in the spleen following a blood-stage Plasmodium chabaudi infection in mice, J Parasitol Res 2011, 534697. https://doi.org/10.1155/2011/534697. [ Links ]
Brown, G.V., 1999, Progress in the development of malaria vaccines: context and constraints, Parassitologia 41, 429-32. [ Links ]
Casals-Pascual, C., Kai, O., Cheung, J.O., et al, 2006, Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo, Blood 108, 2569-77. https://doi.org/10.1182/blood-2006-05-018697. [ Links ]
Chang, K.H., Stevenson, M.M., 2004, Malarial anaemia: mechanisms and implications of insufficient erythropoiesis during blood-stage malaria, Int J Parasitol 34, 1501-16. https://doi.org/10.1016/j.ijpara.2004.10.008. [ Links ]
Chow, L., Soontararak, S., Wheat, W., et al., 2022, Canine polarized macrophages express distinct functional and transcriptomic profiles, Frontiers in Veterinary Science 9, 988981. https://doi.org/10.3389/fvets.2022.988981. [ Links ]
Collett, M.G., 2000, Survey of canine babesiosis in South Africa, J S Afr Vet Assoc 71, 180-6. https://doi.org/10.4102/jsava.v71i3.710. [ Links ]
Corbett, Y., Parapini, S., Perego, F., et al., 2021, Phagocytosis and activation of bone marrow-derived macrophages by Plasmodium falciparum gametocytes, Malaria Journal 20, 81-81. https://doi.org/10.1186/s12936-021-03589-2. [ Links ]
Cowgill, E.S., Neels, J.A., Grindem, C.B., 2003, Clinical application of reticulocyte counts in dogs and cats, Veterinary Clinics of North America Small Animal Practice 33, 1223-1244. https://doi.org/10.1016/S0195-5616(03)00099-8. [ Links ]
Dank, G., Segev, K., Elazari, M., et al., 2020, Diagnostic and prognostic significance of rubricytosis in dogs: A retrospective case-control study of 380 cases, Israel Journal of Veterinary Medicine 75, 193-203. [ Links ]
De Villiers, L., Quan, M., Troskie, M., et al., 2020, A comparison between manual count, flow cytometry and quantitative real-time polymerase chain reaction as a means of determining Babesia rossi parasitaemia in naturally infected dogs, Acta Parasitol 65, 128-135. https://doi.org/10.2478/s11686-019-00134-9. [ Links ]
Elmore, S.A., 2006, Enhanced histopathology of the bone marrow, Toxicol Pathol 34, 666-686. https://doi.org/10.1080/01926230600939971. [ Links ]
Fell, A.H., Smith, N.C., 1998, Immunity to asexual blood stages of plasmodium: is resistance to acute malaria adaptive or innate? Parasitology Today 14, 364-369. https://doi.org/10.1016/S0169-4758(98)01298-8. [ Links ]
Fleming, A.F., 1989, The aetiology of severe anaemia in pregnancy in Ndola, Zambia, Ann Trop Med Parasitol 83, 37-49. https://doi.org/10.1080/00034983.1989.11812309. [ Links ]
Goddard, A., Leisewitz, A.L., Kjelgaard-Hansen, M., et al., 2016, Excessive pro-inflammatory serum cytokine concentrations in virulent canine babesiosis, PLoS One 11, e0150113. https://doi.org/10.1371/journal.pone.0150113. [ Links ]
Goddard, A., Leisewitz, A.L., Kristensen, A.T. et al., 2015a, Platelet activation and platelet-leukocyte interaction in dogs naturally infected with Babesia rossi, Vet J 205, 387-92. https://doi.org/10.1016/j.tvjl.2015.05.008. [ Links ]
Goddard, A., Leisewitz, A.L., Kristensen, A.T. et al., 2015b, Platelet indices in dogs with Babesia rossi infection, Vet Clin Pathol 44, 493-7. https://doi.org/10.1111/vcp.12306. [ Links ]
Goddard, A., Wiinberg, B., Schoeman, J.P., et al., 2013, Mortality in virulent canine babesiosis is associated with a consumptive coagulopathy, Vet J 196, 213-7. https://doi.org/10.1016/j.tvjl.2012.09.009. [ Links ]
Grun, J.L., Weidanz, W.P., 1981, Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse, Nature 290, 143-145. https://doi.org/10.1038/290143a0. [ Links ]
Haddad, J.L.R.S.C., Grindem, C.B., 2020, Cowell and Tyler's Diagnostic Cytology and Hematology of the Dog and Cat. [ Links ]
Harvey, J.W., 2012, Evaluation of erythrocytes, Veterinary Hematology 49-121. https://doi.org/10.1016/B978-1-4377-0173-9.00004-X. [ Links ]
Henning, A., Clift, S.J., Leisewitz, A.L., 2020, The pathology of the spleen in lethal canine babesiosis caused by Babesia rossi, Parasite Immunol 42, e12706. https://doi.org/10.1111/pim.12706. [ Links ]
Hossain, M.A., Yamato, O., Kim, G., et a., 2007, Suppressive effect of culture supernatant of erythrocytes and serum from dogs infected with Babesia gibsoni on the morphological maturation of canine reticulocytes in vitro, Journal of Veterinary Science 8, 169-169. https://doi.org/10.4142/jvs.2007.8.2.169. [ Links ]
Ince, C., Mayeux, P.R., Nguyen, T., et al., 2016, The endothelium in sepsis, Shock 45, 259-70. https://doi.org/10.1097/SHK.0000000000000473. [ Links ]
Jacobson, L.S., 2006, The South African form of severe and complicated canine babesiosis: clinical advances 1994-2004, Vet Parasitol 138, 126-39. https://doi.org/10.1016/j.vetpar.2006.01.047. [ Links ]
Kettner, F., Reyers, F., Miller, D., 2003, Thrombocytopenia in canine babesiosis and its clinical usefulness, J S Afr Vet Assoc 74, 63-8. https://doi.org/10.4102/jsava.v74i3.512. [ Links ]
Kirtz, G., Leschnik, M., Hooijberg, E., et al., 2012, In-clinic laboratory diagnosis of canine babesiosis (Babesia canis canis) for veterinary practitioners in Central Europe, Tierarztl Prax Ausg KKleintiere Heimtiere 40, 87-94. https://doi.org/10.1055/s-0038-1623628. [ Links ]
Knuttgen, H.J., 1987, The bone marrow of non-immune Europeans in acute malaria infection: a topical review, Ann Trop Med Parasitol 81, 567-576. https://doi.org/10.1080/00034983.1987.11812158. [ Links ]
Kueh, Y.K., Yeo, K.L., 1982, Haematological alterations in acute malaria, Scand J Haematol 29, 147-152. https://doi.org/10.1111/j.1600-0609.1982.tb00576.x. [ Links ]
Kurtzhals, J.A., Rodrigues, O., Addae, M., et al., 1997, Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparum malaria, Br J Haematol 97, 169-174. https://doi.org/10.1046/j.1365-2141.1997.82654.x. [ Links ]
Laurencet, F.M., Martinez, T., Beris, P., 1997, Spurious extreme reticulocytosis with an automated reticulocyte analyzer, New England Journal of Medicine 337, 1922-1923. https://doi.org/10.1056/NEJM199712253372615. [ Links ]
Leisewitz, A.L., Goddard, A., Clift, S., et al., 2019, A clinical and pathological description of 320 cases of naturally acquired Babesia rossi infection in dogs, Vet Parasitol 271, 22-30. https://doi.org/10.1016/j.vetpar.2019.06.005. [ Links ]
Liebenberg, C., Goddard, A., Wiinberg, B., et al., 2013, Hemostatic abnormalities in uncomplicated babesiosis (Babesia rossi) in dogs, J Vet Intern Med 27, 150-6. https://doi.org/10.1111/jvim.12016. [ Links ]
Lucidi, C.A., De Rezende, C.L.E., Jutkowitz, L.A., et al., 2017, Histologic and cytologic bone marrow findings in dogs with suspected precursor-targeted immune-mediated anemia and associated phagocytosis of erythroid precursors, Vet Clin Pathol 46, 401-415. https://doi.org/10.1111/vcp.12502. [ Links ]
Mandell, C.P., Jain, N.C., Farver, T.B., 1989, The significance of normoblastemia and leukoerythroblastic reaction in the dog, The Journal of the American Animal Hospital Association 26, 665-672. [ Links ]
Martin, C., Clift, S., Leisewitz, A. 2023. Lung pathology of natural Babesia rossi infection in dogs, J S Afr Vet Assoc 2023, 91, 59-68. [ Links ]
Matjila, P.T., Leisewitz, A.L., Jongejan, F., et al., 2008, Molecular detection of tick-borne protozoal and ehrlichial infections in domestic dogs in South Africa, Vet Parasitol 155, 152-7. https://doi.org/10.1016/j.vetpar.2008.04.012. [ Links ]
Mohan, K., Stevenson, M.M., 1998, Dyserythropoiesis and severe anaemia associated with malaria correlate with deficient interleukin-12 production, Br J Haematol 103, 942-949. https://doi.org/10.1046/j.1365-2141.1998.01126.x. [ Links ]
Nolte, A., Junginger, J., Baum, B., et al., 2017, Heterogeneity of macrophages in canine histiocytic ulcerative colitis, Innate immunity 23, 228-239. https://doi.org/10.1177/1753425916686170. [ Links ]
Onishi, T., Suzuki, S., Horie, M., et al., 1993, Serum hemolytic activity of Babesia gibsoni-infected dogs: the difference in the activity between self and nonself red blood cells, J Vet Med Sci 55, 203-6. https://doi.org/10.1292/jvms.55.203. [ Links ]
Paim, C.B., Paim, F.C., Da Silva, A.S., et al., 2012, Thrombocytopenia and platelet activity in dogs experimentally infected with Rangelia vitalii, Veterinary Parasitology 185, 131-7. https://doi.org/10.1016/j.vetpar.2011.09.039. [ Links ]
Parmar, J., Sheikh, S., 2015, Study of bone marrow: dyserythropoiesis for etiological evaluation of anemia, International Journal of Research in Medical Sciences 3734-3738. https://doi.org/10.18203/2320-6012.ijrms20151431. [ Links ]
Pathak, V.A., Ghosh, K., 2016, Erythropoiesis in malaria infections and factors modifying the erythropoietic response, Anemia 2016, 9310905. https://doi.org/10.1155/2016/9310905. [ Links ]
Pawsat, G.A., Fry, M.M., Behling-Kelly, E., et al., 2023, Bone marrow iron scoring in healthy and clinically ill dogs with and without evidence of iron-restricted erythropoiesis, Veterinary Clinical Pathology 52, 243-251. https://doi.org/10.1111/vcp.13209. [ Links ]
Penzhorn, B.L., 2011, Why is Southern African canine babesiosis so virulent? An evolutionary perspective, Parasit Vectors 4, 51. https://doi.org/10.1186/1756-3305-4-51. [ Links ]
Piane, L., Théron, M.L., Aumann, M., et al., 2016, Spurious reticulocyte profiles in a dog with babesiosis, Veterinary Clinical Pathology 45, 594-597. https://doi.org/10.1111/vcp.12395. [ Links ]
Rautenbach, Y., Goddard, A., Thompson, P.N., et al., 2017, A flow cytometric assessment of the lymphocyte immunophenotypes in dogs naturally infected with Babesia rossi, Veterinary Parasitology 241, 26-34. https://doi.org/10.1016/j.vetpar.2017.05.001. [ Links ]
Schaefer, D.M., Stokol, T., 2016, Retrospective study of reticulocyte indices as indicators of iron-restricted erythropoiesis in dogs with immune-mediated hemolytic anemia, Journal of Veterinary Diagnostic Investigation 28, 304-308. https://doi.org/10.1177/1040638715618231. [ Links ]
Scheepers, E., Leisewitz, A.L., Thompson, P.N., et al., 2011, Serial haematology results in transfused and non-transfused dogs naturally infected with Babesia rossi, J S Afr Vet Assoc 82, 136-43. https://doi.org/10.4102/jsava.v82i3.51. [ Links ]
Seejarim, C., Rautenbach, Y., Hooijberg, E.H., et al., 2023, Regenerative response in dogs naturally and experimentally infected with Babesia rossi, Vet Clin Pathol 52, 422-432. https://doi.org/10.1111/vcp.13228. [ Links ]
Seung, B.-J., Lim, H.-Y., Shin, J.-I., et al., 2018, CD204-expressing tumor-associated macrophages are associated with malignant, high-grade, and hormone receptor-negative canine mammary gland tumors, Veterinary Pathology 55, 417-424. https://doi.org/10.1177/0300985817750457. [ Links ]
Sharma, D., Priest, H., Wilcox, A., 2022, Pseudoreticulocytosis by the ADVIA 2120 hematology analyzer and other hematologic changes in a cynomolgus macaque (Macaca fascicularis) with Malaria, Toxicologic Pathology 50, 684-692. https://doi.org/10.1177/01926233221083217. [ Links ]
Sheikha, A., 2004, Dyserythropoiesis in 105 patients with visceral leishmaniasis, Laboratory hematology: official publication of the International Society for Laboratory Hematology 10, 206-211. [ Links ]
Skudowitz, R.B., Katz, J., Lurie, A., et al., 1973, Mechanisms of thrombocytopenia in malignant tertian malaria, Br Med J 2, 515-518. https://doi.org/10.1136/bmj.2.5865.515. [ Links ]
Smith, R.L., Goddard, A., Boddapati, A., et al., 2021, Experimental Babesia rossi infection induces hemolytic, metabolic, and viral response pathways in the canine host, BMCgenomics 22, 1-16. https://doi.org/10.1186/s12864-021-07889-4. [ Links ]
Spotswood, T.C., Kirberger, R.M., Koma, L.M.P.K., et al., 2005, A canine model of normovolaemic acute anaemia, Onderstepoort J Vet Res 72. https://doi.org/10.4102/ojvr.v72i2.209. [ Links ]
Stockham, S.L., Scott, M.A., 2008, Fundamentals of Veterinary Clinical Pathology, Blackwell Publishing. [ Links ]
Travlos, G.S., 2006, Histopathology of bone marrow, Toxicol Pathol 34, 566-598. https://doi.org/10.1080/01926230600964706. [ Links ]
Tvedten, H., 2022, Classification and laboratory evaluation of anemia, Schalm's Veterinary Hematology 198-208. https://doi.org/10.1002/9781119500537.ch25. [ Links ]
Van Zyl, E., Leisewitz, A.L., Atkinson, B.K., et al., 2022, Serial changes in the concentrations of cortisol and thyroid hormones in Beagle dogs infected with Babesia rossi, Ticks Tick Borne Dis 14, 102107. https://doi.org/10.1016/j.ttbdis.2022.102107. [ Links ]
Weatherall, D.J., Abdalla, S., Pippard, M.J., 1983, The anaemia of Plasmodium falciparum malaria, Ciba Found Symp 94, 74-97. https://doi.org/10.1002/9780470715444.ch6. [ Links ]
Weiss, G., Ganz, T., Goodnough, L.T., 2019, Anemia of inflammation, Blood 133, 40-50. https://doi.org/10.1182/blood-2018-06-856500. [ Links ]
Weiss, G.E., Crompton, P.D., Li, S., et al., 2009, Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area, The Journal of Immunology 183, 2176-2182. https://doi.org/10.4049/jimmunol.0901297. [ Links ]
Welzl, C., Leisewitz, A.L., Jacobson, L.S., et al., 2001, Systemic inflammatory response syndrome and multiple-organ damage/dysfunction in complicated canine babesiosis, J S Afr Vet Assoc 72, 158-62. https://doi.org/10.4102/jsava.v72i3.640. [ Links ]
White, N.J., Ho, M., 1992, The pathophysiology of malaria, Adv Parasitol 31, 83-173. https://doi.org/10.1016/S0065-308X(08)60021-4 [ Links ]
Wickramasinghe, S.N., Abdalla, S.H., 2000, Blood and bone marrow changes in malaria, Baillieres Best Pract Res Clin Haematol 13, 277-99. https://doi.org/10.1053/beha.1999.0072 [ Links ]
Wickramasinghe, S.N., Looareesuwan, S., Nagachinta, B., et al., 1989, Dyserythropoiesis and ineffective erythropoiesis in Plasmodium vivax malaria, Br J Haematol 72, 91-99. https://doi.org/10.1111/j.1365-2141.1989.tb07658.x [ Links ]
Xu, L., Zheng, X., Berzins, K., et al., 2013, Cytokine dysregulation associated with malarial anemia in Plasmodium yoelii infected mice., American Journal of Translational Research 5, 235-245. [ Links ]
 Correspondence:
Correspondence:
AL Leisewitz
Email: all0087@auburn.edu













