Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Southern African Journal of Critical Care (Online)
On-line version ISSN 2078-676XPrint version ISSN 1562-8264
South. Afr. j. crit. care (Online) vol.31 n.1 Pretoria Jun. 2015
https://doi.org/10.7196/SAJCC.202
ARTICLE
Influenza A(H1N1)pdm09 in critically ill children admitted to a paediatric intensive care unit, South Africa
J O AhrensI, II; B M MorrowIII; A C ArgentIV, V
IFCPaeds (SA); Paediatric Intensive Care Unit, Red Cross War Memorial Children's Hospital, Cape Town, South Africa
IIFCPaeds (SA); Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
IIIPhD; Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
IVMD, FCPaeds (SA); Paediatric Intensive Care Unit, Red Cross War Memorial Children's Hospital, Cape Town, South Africa
VMD, FCPaeds (SA); Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
ABSTRACT
OBJECTIVE: To describe the clinical course of critically ill children with confirmed pandemic influenza A(H1N1)pdm09 (H1N1) infection in a southern African paediatric intensive care unit (PICU), and to compare them with a similar group with respiratory virus infections other than H1N1 admitted to the same PICU during the same period.
METHODS: A retrospective descriptive study of all patients admitted to a PICU in Cape Town, South Africa, who tested positive for H1N1 and other respiratory viruses from 1 August to 30 September 2009.
RESULTS: A total of 19 children in 20 PICU admissions tested positive for H1N1 (Group 1). Of these, 14 (70%) had major comorbidities and 4 tested positive for another respiratory virus. Five (26.3%) children in this group died and seven (36.8%) had nosocomial infection. Eight patients in nine PICU admissions who tested H1N1-negative (Group 2), tested positive for other respiratory viruses. Of these, five (55.6%) had major comorbidities. None in this group died. Children in Group 1 had significantly longer ICU stays, ventilator days and worse indices of organ dysfunction than those in Group 2.
CONCLUSIONS: Children admitted to the PICU with confirmed H1N1 tended to have longer ICU stays, prolonged ventilation, more severe organ dysfunction and higher mortality than those with other respiratory viruses. Hospitalisation was identified as a major risk factor for chronically ill children to acquire H1N1 infection requiring intensive care in our setting.
The first case of 'swine flu' (pandemic influenza A(H1N1) pdm09 (H1N1)) in South Africa (SA) was diagnosed on 17 June 2009.[1] Red Cross War Memorial Children's Hospital (RCWMCH) in Cape Town, SA, was part of a sentinel epidemiological testing site to monitor the pandemic in SA. The first case of H1 N1 at RCWMCH was confirmed on 3 August 2009, following which a further 92 children at RCWMCH tested positive for the virus until the end of September 2009.
Surveillance data of the 2009 H1N1 epidemic in SA supplied by the SA National Institute of Communicable Diseases[2] show that there were 12 636 laboratory-confirmed cases during the period May - December 2009, with the peak incidence in early August and a rapid decline towards the end of September 2009. The incidence rate of laboratory-confirmed cases was 25.6 and 39.5 per 100 000 population in SA and the Western Cape Province, respectively. There were 93 recorded deaths associated with the outbreak nationally (about 0.7%). The weekly incidence of laboratory-confirmed cases at RCWMCH was similar to the national trend (Fig. 1).
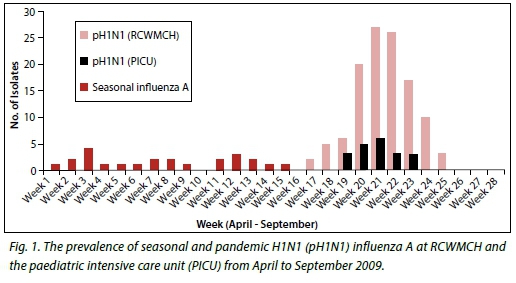
Early studies of critically ill children with H1N1 infection from northern[3-9] and southern hemisphere[10-13] countries showed that younger children and those with preexisting chronic medical conditions were at greater risk of more severe disease, including shock, multi-organ failure and higher mortality.
The initial wave of the 2009 flu pandemic in the northern hemisphere countries took place during their spring and summer months, which coincided with the end of their seasonal influenza season, whereas in the southern hemisphere the epidemic started in the winter months and coincided with the seasonal outbreaks of other respiratory viral infections, including seasonal influenza. Few data exist as to what extent the clinical picture of H1N1 differed from that of the seasonal respiratory virus infections in children, and what the effect of the 2009 flu pandemic was on paediatric critical care resources in southern Africa. Such data would help in planning optimal use of paediatric critical care resources in a country with limited resources and limited ability to test for respiratory virus infections, leaving clinicians to make decisions about clinical care and resource allocation purely on clinical grounds rather than in conjunction with laboratory testing.
This was the first study of critically ill children with H1N1 infection in southern Africa.
Objectives
The objectives of this study were to describe the clinical course of children managed in the paediatric intensive care unit (PICU) at RCWMCH with laboratory-confirmed H1N1 infection, and to compare the clinical course of these children with a cohort of other respiratory viral isolates admitted to the PICU during the same period.
Methods
This was a retrospective descriptive study of all children admitted to the PICU at RCWMCH from 1August to 30 September 2009, who tested positive for the following respiratory viruses: H1N1, seasonal influenza A, influenza B, parainfluenza 1/2/3, rhinovirus, respiratory syncytial virus (RSV), human metapneumovirus (HMPV) and adenovirus.
Patients were tested for the above respiratory viruses if they met the clinical case definition for a moderate or severe acute respiratory infection (SARI),[14] had household contact with suspected H1N1 infection, or at the discretion of the PICU consultant. The children were divided into two groups, Group 1 being those who tested positive for the H1N1 virus, and Group 2 those who tested positive for respiratory viruses other than H1N1.
Non-directed bronchial alveolar lavage specimens, tracheal aspirates or nasopharyngeal aspirates obtained as part of standard practice were submitted to the virology laboratory of the National Health Laboratory Services at Groote Schuur Hospital, Cape Town, for detection of respiratory viruses. Initially samples were tested using the Multiplex 7 virus polymerase chain reaction (PCR) (Seeplex RV detection kit, Seegene, Seoul, Korea), with influenza A-positive samples typed with supplementary primers included in the kit. In mid-August 2009, testing was changed to H1N1-specific screening using an Advanced Realtime PCR kit (Luminex Molecular Diagnostics, Inc., Toronto, Canada) for Swine H1N1 influenza. Negatives were tested by respiratory viral PCR (RV-PCR) the following day. In mid-September 2009, the real-time screening was stopped and testing was resumed with RV-PCR.
Every patient admitted to the PICU with a SARI or proven H1N1 infection during the study period was given a 5-day course of the neuraminidase inhibitor, oseltamivir, which was extended in some patients who had prolonged illness and who continued to test positive for the H1N1 virus.
The following data were collected and recorded on an Excel 2007 (Microsoft Corporation, USA) spreadsheet: patient demographics (age, gender, address), clinical observations (temperature, weight, nutritional status, daily fluid balance, primary diagnoses and comorbidities), treatment (including antimicrobial treatment, oseltamivir, corticosteroids, diuretics, mode of respiratory support and use of inotropes), special investigations (arterial blood gases, blood electrolytes, full blood count and liver function tests, tests for respiratory viruses and bacterial co-infections and inflammatory markers), measures of oxygenation (oxygenation index, PaO2:FiO2 ratio on admission), Pediatric Index of Mortality Score (PIM2) on admission, daily Pediatric Logistic Organ Dysfunction (PELOD) score, duration of PICU stay and mechanical ventilation, and PICU mortality. Extracorporeal membrane oxygenation was not available.
Full approval for the study was obtained from the Human Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town. The study adhered to the provisions laid down in the Declaration of Helsinki (2013).[15]
Analysis
Data were tested for normality using Kolmogorov Smirnov and Lilliefors tests. Data were not normally distributed and were therefore presented as median (interquartile range (IQR)) or proportions for categorical variables. Chi-square tests (for categorical data), with Yates correction for cells values <10, and the Mann-Whitney U-test (for continuous data) were used for comparisons between Groups 1 and 2, and between survivors and non-survivors in Group 1. The Kruskal-Wallis analysis of variance (ANOVA) by ranks was used to assess differences among multiple variables. Statistica version 10 (2011) (StatSoft Inc., USA) was used for data analysis. A significance level of p<0.05 was chosen.
Results
Nineteen children in 20 PICU admissions tested positive for H1N1 during the study period (Group 1), comprising 20.2% of the total H1N1-positive children presenting to RCWMCH (both in- and outpatients).
Three patients in Group 1 were co-infected with rhinovirus, and one was co-infected with adenovirus. Eight children in nine PICU admissions tested positive for a respiratory virus other than H1N1 (Group 2), namely: rhinovirus (n=4, 50%); rhinovirus plus influenza B (n=1), rhinovirus plus seasonal influenza A (n=1, 12.5%), RSV plus seasonal influenza A (n=1, 12.5%) and adenovirus alone (n=1, 12.5%).
Table 1 summarises the clinical features of patients in Group 1 and Group 2, and also compares survivors and non-survivors in Group 1. The median age of H1N1-infected children was 12 months, with only three patients older than three years. Both sexes were equally prone to severe H1N1 infection. Despite seven of the eight patients in Group 2 being male, the difference in gender distribution between groups did not reach statistical significance (Table 1).
In Group 1, the underlying reasons precipitating PICU admission were respiratory failure from pneumonia (n=14), elective cardiac surgery (n=3), laryngotracheobronchitis (n=1), severe burns (n=1) and trauma from a motor vehicle accident (n=1). The reasons for PICU admission of the children in Group 2 were pneumonia (n=3), laryngotracheobronchitis (n=3), Guillain-Barre syndrome (n=1), septic shock in a child who had a previous liver transplant (n=1) and seizures and obstructive hydrocephalus from tuberculous meningitis (n=1). Both groups of patients had a high incidence of comorbidities. The rate of HIV and bacterial co-infections was not significantly different between the two groups (Table 1).
Seven children (36.8%) in Group 1 had a presumed nosocomially acquired respiratory virus infection, defined as having first evidence (clinically or by laboratory confirmation) of a respiratory virus infection at least 72 hours after hospital admission, whereas none of the patients in Group 2 acquired infection nosocomially (p<0.05) (Table 1). Of the seven who likely acquired the H1N1 infection nosocomially, one was transferred from a regional hospital for PICU care. Three others had been admitted to the cardiac ward awaiting cardiac surgery, where pre- and postoperative cardiac surgery patients co-habited. They had no preoperative symptoms or signs of a respiratory virus infection, had not received prophylactic oseltamivir, underwent elective cardiac surgery and had a postoperative course in the PICU complicated by pneumonia, with prolonged periods of ventilation and PICU stay. All three had bacterial co-infections. Three other children with chronic medical conditions (end-stage renal failure, chronic lung disease, epilepsy) had been inpatients in general paediatric wards for long-term care where no isolation facilities were available to protect patients at risk from nosocomial infections.
Indices of illness severity and multi-organ involvement were higher in Group 1 than in Group 2 on admission, with median admission PELOD score and serum lactate 11 v. 1 (p=0.02) and 1.1 v. 0.8 (p=0.02), respectively (Table 1). A similar percentage of patients in both groups required invasive ventilation and, although patients in Group 1 had longer durations of ventilation than patients in Group 2 (5 v. 1 median
ventilator days), this was not statistically significant (Table 1). Of clinical importance was that patients in Group 1 had worse indices of oxygenation on day 3 of ventilation than those in Group 2, as suggested by higher median oxygenation index (OI) (6.07 v. 1.98; p=0.008) and lower median PaO2:FIO2 ratio (170.16 v. 306.67; p=0.002) (Table 1). The predicted PICU mortality according to the median PIM2 scores was similar for both groups (Table 1). Five (26.3%) children infected with H1N1 died: three as a result of respiratory failure, and two from overwhelming sepsis (Table 2). Four of the patients who died had major comorbidities (Table 2). No deaths occurred in Group 2 (p=0.3). There were no significant differences between survivors and non-survivors in Group 1 (Table 1).
Discussion
This is the first study from SA to describe children severely affected by the 2009 H1N1 pandemic and who required PICU admission. The RCWMCH PICU admission rate (22%) for H1N1-positive children was comparable with the 9.3 - 26.0% reported from Canada[7] and the USA[16] during the outbreak.
Although small patient numbers precluded identification of risk factors for H1N1 infection or mortality, it is notable that 80% of critically ill children with H1N1 had major comorbid conditions, including HIV infection (Table 1). Other centres around the world have also reported that children with comorbidities such as asthma and neurological or developmental conditions were at particular risk of severe H1N1 disease, and at greater risk of dying from H1N1 infection.[16,17] Bacterial co-infection has been reported as a major risk factor for mortality in studies from nearly every major influenza pandemic, including the 2009 pandemic.[5,11,16] A high proportion of our patients, particularly in the H1N1-positive group, had documented or suspected bacterial co-infection (Table 1).
The H1N1 outbreak had an immediate effect on the availability of PICU beds for elective surgery during August 2009. In accordance with the hospital's escalation plan, all elective surgery cases requiring postoperative PICU admission were cancelled for a 2-week period during the peak of the pandemic in the Western Cape, during which time period the PICU ran at 100% occupancy. Fortunately, the number of H1N1-positive patients declined rapidly during September 2009 and the elective surgery list could return to normal. Some other centres have also commented that the pandemic placed strain on critical care resources.[18,19]
Patients with H1N1 infection admitted to the PICU had more severe organ dysfunction and disease severity compared with the H1N1-negative group, as evidenced by higher admission PELOD scores and poorer oxygenation on day 3. Although clinical outcome measures were not found to be statistically different, children in the H1N1 group had clinically significantly longer durations of PICU stay and mechanical ventilation, as well as higher mortality. Observations from other countries have been varied, with some countries reporting higher mortality rates and more severe illness among critically ill children with p(H1N1) disease,[5,18-20] whereas some countries have reported lower disease acuity and mortality rates than expected, particularly if compared with the previous seasonal influenza seasons.[21,22]
The mortality rate of children with H1N1 was 26.3%, whereas mortality rates in other PICUs have been between 0% (Netherlands)[23] and 39% (Argentina).[18] Of the children who died, four had major underlying comorbidities and none tested positive for any other respiratory virus.
The high rate of presumed nosocomially acquired H1N1 infection is concerning, but not unexpected in the context of lack of isolation and cohorting facilities, and the fact that an effective vaccine was not yet available in our hospital at the outbreak of the epidemic. The neuraminidase inhibitor oseltamivir also only became available for prophylaxis for exposed H1N1 contacts half-way through the epidemic. Once available, four of the children admitted to PICU had received oseltamivir prior to PICU admission, and all four survived to discharge.
Conclusion
Children admitted to an SA PICU with H1N1 infection had evidence of more severe inflammatory disease, greater organ dysfunction and worse respiratory indices, and had poorer outcome in terms of PICU stay and mortality rate compared with children admitted to the PICU with other respiratory viruses. Comorbities and co-infection with bacterial pathogens were common. These findings are similar to those of other studies of critically ill children in other centres in the world, particularly from the southern hemisphere, relating to the 2009 swine flu pandemic.
This study also highlights the problem of hospital-acquired pandemic influenza infections in a setting of a tertiary hospital with a high bed occupancy rate, emphasising the potential for acquisition of these viruses by hospitalised children with significant comorbidities. Attention must be paid to implementing appropriate infection control procedures in order to prevent cross-infection in case of future pandemics.
References
1. Schoub B. Swine flu - implications for South Africa. Commun Dis Surveill Bull 2009;7(3):5-7. [ Links ]
2. National Institute for Communicable Diseases of the National Health Laboratory Services. Situation Update Pandemic Influenza A(H1N1) 2009, South Africa. Report no. SWIN110809 SITREP. Johannesburg: NICD, 2009. http://www.nicd.ac.za (accessed 15 August 2015). [ Links ]
3. Hackett S, Hill L, Patel J, et al. Clinical characteristics of paediatric H1N1 admissions in Birmingham, UK. Lancet 2009;374(9690):61511-61517. [http://dx.doi.org/10.1016/S0140-6736(09)61511-7] [ Links ]
4. Lister P, Reynolds F, Parslow R, et al. Swine-origin influenza virus H1N1, seasonal influenza virus, and critical illness in children. Lancet 2009;374(9690):605-607. [http://dx.doi.org/10.1016/S0140-6736(09)61512-9] [ Links ]
5. Lockman JL, Fischer WA, Perl TM, Valsamakis A, Nichols DG. The critically ill child with novel H1N1 influenza A: A case series. Pediatr Crit Care Med 2010;11(2):173-178. [http://dx.doi.org/10.1097/PCC.0b013e3181ccedae] [ Links ]
6. Koliou M, Soteriades ES, Toumasi MM, Demosthenous A, Hadjidemetriou A. Epidemiological and clinical characteristics of influenza A(H1N1)v infection in children: The first 45 cases in Cyprus, June - August 2009. Euro Surveill 2009;14(33):19312. [ Links ]
7. Jouvet P, Hutchison J, Pinto R, et al. Critical illness in children with influenza A/pH1N1 2009 infection in Canada. Pediatr Crit Care Med 2010;11(5):603-609. [http://dx.doi.org/10.1097/PCC.0b013e3181d9c80b] [ Links ]
8. Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April - June 2009. N Engl J Med 2009;361(20):1935-1944. [http://dx.doi.org/10.1056/NEJMoa0906695] [ Links ]
9. Gilsdorf A, Poggensee G, Working Group Pandemic Influenza A(H1N1)v Influenza A(H1N1)v in Germany: The first 10 000 cases. Euro Surveill 2009;14(34):19318. [ Links ]
10. Gomez J, Munayco C, Arrasco J, et al. Pandemic influenza in a southern hemisphere setting: The experience in Peru from May to September, 2009. Euro Surveill 2009;14(42):19371. [ Links ]
11. ANZIC Influenza Investigators, Webb SA, Pettila V, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 2009;361(20):1925-1934. [http://dx.doi.org/10.1056/NEJMoa0908481] [ Links ]
12. Oliveira W, Carmo E, Penna G, et al. Pandemic H1N1 influenza in Brazil: Analysis of the first 34 506 notified cases of influenza-like illness with severe acute respiratory infection (SARI). Euro Surveill 2009;14(42):19362. [ Links ]
13. Archer BN, Timothy GA, Cohen C, et al. Introduction of 2009 pandemic influenza A virus subtype H1N1 into South Africa: Clinical presentation, epidemiology, and transmissibility of the first 100 cases. J Infect Dis 2012;206 (Suppl 1):S148-S153. [http://dx.doi.org/10.1093/infdis/jis583] [ Links ]
14. The National Institute for Communicable Diseases (NICD) of the National Health Laboratory Service (NHLS). Revised Health Workers Handbook on Pandemic Influenza A (H1N1) 'swine flu, 2009. http://www.kznhealth.gov.za/h1n1handbook.pdf (accessed 15 August 2015). [ Links ]
15. World Medical Association (WMA). WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects, 2013. http://www.wma.net/en/30publications/10policies/b3/17c.pdf (accessed 15 August 2015). [ Links ]
16. Randolph AG, Vaughn F, Sullivan R, et al. Critically ill children during the 2009 - 2010 influenza pandemic in the United States. Pediatrics 2011;128(6):e1450-e1458. [http://dx.doi.org/10.1542/peds.2011-0774] [ Links ]
17. Libster R, Bugna J, Coviello S, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med 2010;362(1):45-55. [http://dx.doi.org/10.1056/NEJMoa0907673] [ Links ]
18. Farias JA, Fernandez A, Monteverde E, et al. Critically ill infants and children with influenza A (H1N1) in pediatric intensive care units in Argentina. Intensive Care Med 2010;36(6):1015- 1022. [http://dx.doi.org/10.1007/s00134-010-1853-1] [ Links ]
19. Torres SF, Iolster T, Schnitzler EJ, et al. High mortality in patients with influenza A pH1N1 2009 admitted to a pediatric intensive care unit: A predictive model of mortality. Pediatr Crit Care Med 2012;13(2):e78-83. [http://dx.doi.org/10.1097/PCC.0b013e318219266b] [ Links ]
20. Kendirli T, Demirkol D, Yildizdas D, et al. Critically ill children with pandemic influenza (H1N1) in pediatric intensive care units in Turkey. Pediatr Crit Care Med 2012;13(1):e11-e17. [http://dx.doi.org/10.1097/PCC.0b013e31820aba37] [ Links ]
21. Morgan CI, Hobson MJ, Seger B, Rice MA, Staat MA, Wheeler DS. 2009 pandemic influenza A (H1N1) in critically ill children in Cincinnati, Ohio. Pediatr Crit Care Med 2012;13(3):e140-e144. [http://dx.doi.org/10.1097/PCC.0b013e318228845f] [ Links ]
22. Baird JS, Buet A, Hymes SR, et al. Comparing the clinical severity of the first versus second wave of 2009 influenza A (H1N1) in a New York city pediatric healthcare facility. Pediatr Crit Care Med 2012;13(4):375-380. [http://dx.doi.org/10.1097/PCC.0b013e31823893df] [ Links ]
23. Augustyn B. Ventilator-associated pneumonia: Risk factors and prevention. Crit Care Nurse 2007;27(4):38-39. [ Links ]
 Correspondence:
Correspondence:
B M Morrow
brenda.morrow@uct.ac.za













