Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950Print version ISSN 0378-4738
Water SA vol.45 n.3 Pretoria Jul. 2019
https://doi.org/10.17159/wsa/2019.v45.i3.6734
RESEARCH PAPERS
Removal of lead in water using activated carbon prepared from Acacia catechu
K LakshmikandhanI; A RamadeviII, *
IDepartment of Chemistry, CMS College of Engineering and Technology, Coimbatore, India
IIDepartment of Chemistry, Alagappa Chettiar Government College of Engineering and Technology Karaikudi, India
ABSTRACT
The adsorption of Pb (II) on bicarbonate-treated Acacia catechu carbon (BTACC) and commercial activated carbon (CAC) was investigated to assess the possible use of this adsorbent for Pb (II) removal from aqueous solutions. The results obtained from batch studies showed that 98% Pb (II) adsorption for BTACC occurs within 4 h of contact time, within a pH range of 4-10 and a carbon dosage of 100 mg with initial Pb (II) concentration of 10 mg∙L−1, whereas for CAC 52% Pb (II) adsorption occurred within 5 h of contact time and a narrow range of pH 5 and carbon dosage of 100 mg. Adsorption followed pseudo-second-order kinetics for both carbon sources, with the mechanism of adsorption being intra-particle diffusion and film-diffusion. The best fitting adsorption isotherm for both BTACC and CAC were the Langmuir, Freundlich and Temkin models. Surface characteristics were studied using FT-IR, SEM, EDX.
Keywords: Acacia catechu, commercial activated carbon, adsorption isotherms, kinetics
INTRODUCTION
Water contains impurities of various kinds which are dissolved as well as suspended. Heavy metals are major toxic pollutants with serious health effects on humans (Inglezakis et al., 2003; Demirbas, 2008). The toxicity of heavy metals depends on the concentration of metal ions, and the nature of the organism with which it interacts. The heavy metals are the most toxic metals of widespread use in industries such as tanning, electroplating, electronic equipment, manufacturing and chemical processing plants. Lead has been recognized for centuries as a cumulative poison (Hua et al., 2012; Kadirvelu et al., 2001; WHO, 1977).
Health studies done in Poland have linked elevated levels of lead in the environment with retardation and learning disabilities of children (Groffman et al., 1992). Acute lead poisoning in humans causes severe damage to kidneys, the reproductive system, the liver, the brain and central nervous system. The neurotoxicity of lead is well known but the exact mechanisms of its toxicity are not yet understood. Disturbances in glutamate homeostasis of neural tissue and interactions of lead with calcium metabolism have been considered as potential mechanism of neurotoxicity. (Raunio et al., 2001).
Removal of Pb (II) by adsorption using treated granular activated carbon in both batch and column studies has been studied (Goel et al., 2005). Activated carbon developed from tamarind wood by zinc chloride activation was examined for the removal of Pb (II) from wastewater (Jyotikusum et al., 2009). Activated carbon prepared from marine green algae was used for adsorption of Pb (II) ions (Suresh Jeyakumar et al., 2014). Treatment of lead-contaminated water using activated carbon adsorbent from locally available papaya peel bio-waste has also been investigated (Sahar Abbaszadeh et al., 2016).
In the present investigation, out of various non-conventional adsorbents, modified Acacia catechu carbon is studied for its adsorption capacity for lead (II) removal from aqueous solution. This work reports the results of batch studies on the removal of lead (II) from aqueous solution by adsorption, using bicarbonate-treated Acacia catechu carbon (BTACC) as an adsorbent. Various parameters, such as equilibrium time, pH and dosage of adsorbent, and adsorption isotherms were studied. Commercial activated carbon (CAC) procured from the market was used for evaluation purposes.
MATERIALS AND METHODS
Preparation of bicarbonate-treated Acacia catechu carbon (BTACC)
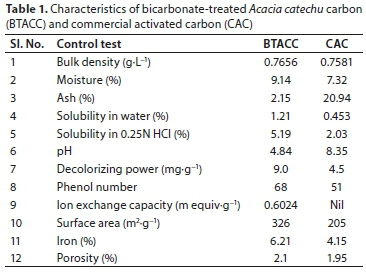
Acacia catechu seeds were collected, washed with distilled water and dried at 110°C. Seeds were then broken mechanically into small particles and sieved to 20-50 ASTM mesh size. Then the material was treated with concentrated sulphuric acid with a weight ratio of 1:1 and kept in an air oven at 150 ± 5° C for 24 h. The carbonized material was washed well with distilled water to remove the free acid and dried at 105 ± 5°C. Then it was soaked in 1% sodium bicarbonate solution until the effervescence ceased, and finally soaked in sodium bicarbonate solution for 24 h to remove any residual acid. The material was then washed with distilled water, and dried at 105 ± 5°C (BTACC). Preliminary studies were carried out with raw Acacia catechu seed, sulphuric acid treated Acacia catechu seed carbon and sulphuric acid treated Acacia catechu seed modified by 1% sodium bicarbonate solution (BTACC) for Pb (II) removal. Based on removal efficiency, BTACC was chosen for further studies. The characteristics of BTACC and commercial activated carbon (CAC) were investigated and are summarized in Table 1. Subsequent experiments were carried out with BTACC and the performance of the carbon was evaluated by using CAC obtained from SD Fine Chemicals, considering the same particle size of 20-50 ASTM.
Batch experiment
A stock solution of Pb (II) was prepared by dissolving 0.3997 g of anhydrous lead nitrate in 100 mL distilled water containing 0.1 mL of concentrated nitric acid (to prevent hydrolysis) and diluting it to 250 mL. This solution was diluted further to 100 mL of Pb (II) solution of desired concentration adjusted to a desired pH in reagent bottles of 300 mL capacity. A known amount of BTACC and CAC were added and pH was adjusted using dilute hydrochloric acid or sodium hydroxide solutions. All chemicals used were of analytical grade and were obtained from Ranbaxy, BDH. The solutions were agitated and the filtrate was analysed for lead content (APHA, AWWA, 1973). Maintaining the dosage of carbon at a constant level, the adsorption isotherm for Pb (II) with different initial concentrations was studied. For pH effect, 10 mg∙L−1 of Pb (II) for BTACC and CAC with a dosage of 100 mg∙100 mL−1 were used. In order to correct for any adsorption of Pb (II) due to the container, control experiments were carried out without adsorbent and there was negligible adsorption by the container wall.
RESULTS AND DISCUSSION
Examination of carbon characteristics in Table 1 shows that the BTACC has higher moisture content than CAC. Higher moisture content of BTACC suggests that the acid treatment made the carbon more porous and BTACC has lower ash content than CAC, which indicates more carbon content. The surface area is found to be greater for BTACC than CAC.
Surface characterization of activated carbon
Fourier transform infrared spectroscopic analysis (FT-IR)
Fourier transform infrared spectroscopy (FT-IR) studies were used to identify the functional groups present on the surface of the adsorbent. FT-IR spectra of BTACC and CAC are shown in Fig. 1a-d. This shows the presence of poly functional groups. The strong absorption peak at 3 445 cm−1, is due to the -OH stretching vibration due to inter- and intra-molecular hydrogen bonding of alcohols, phenols and carboxylic acids. The peaks at 2 928 cm−1, 2 362 cm−1 and 1 698 cm−1 are due to the C-H stretching, C≡C stretching and -CO stretching vibration of ether. The presence of sulphonic acid groups is confirmed by the peak at 1 343 cm−1. From the observations it is evident that some of the peaks shift or become weak indicating the incorporation of heavy metal ion Pb (II) within the adsorbent through the interaction of the active functional group after adsorption.
Scanning electron microscopy (SEM)
SEM was used to study the morphology and surface characteristics of the adsorbent material (Tharanitharan et al., 2009). Adsorption capacity depends on the chemical composition of the adsorbent. The pore size, shape and volume also play an important role in the adsorption process. The SEM images of the adsorbent BTAAC and CAC before and after adsorption of Pb (II) ions are given in Fig. 2a-d. This image shows the presence of isolated pores and uneven cavities before and after adsorption. The irregular and porous surface activated carbon could be observed. On the basis of this fact, it can be concluded that BTACC presents an adequate morphology for Pb (II) adsorption (Deepak et al., 2013).
Energy dispersive X-ray analysis (EDX)
Energy dispersive X-ray analysis (EDX) technique is used for elemental analysis or chemical characterization of a sample in conjunction with scanning electron microscopy (SEM). To resolve the elemental content, the electron-beam strikes the surface of a conducting sample (SEM). The EDX spectra of the Pb (II) ion adsorbed on BTACC and CAC show peaks for metal ions in addition to the other cations, confirming the adsorption of Pb (II) ions on the surface of the adsorbent (Fig. 3 a-d).
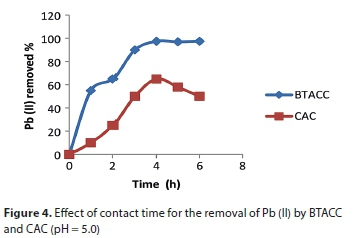
Effect of agitation time
The effect of agitation time on the removal of Pb (II) by BTACC and CAC is shown in Fig. 4. Percentage removal increases with time and attains equilibrium at 3 h for BTACC and 4 h for CAC for an initial concentration of 10 mg∙L−1 of Pb (II). However, 4 h and 5 h shaking time was maintained for BTACC and CAC, respectively, for further studies. It was found that BTACC showed 98% lead (II) removal whereas CAC showed 52% removal for a carbon dosage of 100 mg∙100 mL−1 of Pb (II) ion solution of initial concentration of 10 mg∙L−1.
Effect of carbon dosage
Figure 5 represents the removal of Pb (II) as a function of carbon dosage for both BTACC and CAC. It was shown that a minimum carbon dosage of 100 mg of BTACC was required for 98% removal of Pb (II) from a 10 mg∙L−1 lead solution. However, a maximum removal of 52% was observed for CAC with a carbon dosage of 100 mg for the same concentration of Pb (II). This shows that BTACC was nearly 2 times more efficient than CAC for Pb (II) removal.

Effect of pH
Figure 6 shows the effect of initial pH on the removal of Pb (II) by BTACC and CAC. It is clear that BTACC is effective for the quantitative removal of Pb (II) over the pH range of 4.0-10.0. However, for CAC the quantitative removal was only at a narrow pH of 5. The influence of pH on lead removal can likely be explained as follows. At lower pH, a higher concentration of hydrogen ions present in the mixture competes with the positively charged metal ion for the adsorption sites, resulting in the reduced uptake of metal ions. As the pH increases, the concentration of Pb (II) ions remains constant and therefore the uptake of metal ions can be explained as H+-Pb2+ exchange reaction. The major mechanism of adsorption of Pb2+ seems to be ion exchange. Furthermore, a pure carbon surface is considered to be non-polar, but in actual practice some carbon-oxygen complexes, such as CxO, COx and CxO2 are usually present which render the surface slightly polar. The interaction of the above groups with aqueous phase may lead to the following hydrolytic reactions:

CxO + 2H2O CxOH22+ + 2OH-
COx + XH2O C(OH)x+ + XOH-
CxO2 + H2O CxO2+ + 2OH-
Since BTACC was prepared upon treatment with sulphuric acid and sodium bicarbonate, groups such as CxONa2+, CxONa+, CxSO3H and CxSO3Na are also assumed to be present. Na+ ions in the above groups are also exchanged with H+ in the medium as follows:
CxONa+ + H+ CxO H+ + Na+
CxONa2+ + 2 H+ Cx(OH)2x + Na+
CxSO3Na + H+ CxSO3HNa+
Under these conditions lead ions are expected to exchange as follows:
2CxOH+ + Pb2+ (CxO)2 Pb2+ + 2 H+
CxOH22+ + Pb2+ CxO Pb2+ + 2 H+
2CxONa+ + Pb2+ (CxO)2 Pb2+ + 2 Na+
CxONa22+ + Pb2+ CxO Pb2+ + 2 Na+
2CxSO3H + Pb2+ (CxSO3)2 Pb + 2 H+
2CxSO3Na + Pb2+ (CxSO3)2 Pb + 2 Na+
Adsorption isotherms
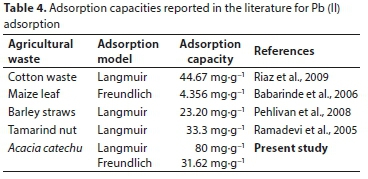
Results for application of various isotherm models for adsorption by BTACC and CAC are given in Table 3. Reported adsorption capacities of various materials for Pb (II) are given in Table 4.

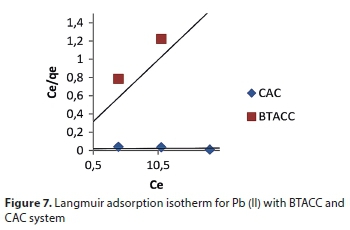
The Langmuir equation was applied for adsorption equilibrium for both BTACC and CAC:

where Ce is equilibrium concentration (mg∙L−1), qe is amount adsorbed at equilibrium (mg∙g−1) and Q0 and b are Langmuir constants related to adsorption capacity and energy of adsorption, respectively (Hall et al., 1966; Webber et al., 1974). The linear plots of Ce/qe vs. Ce showed that the experimental data fitted well and absorption obeys the Langmuir model for both BTACC and CAC (Fig. 7).
The Freundlich isotherm is represented by Eq. 2:
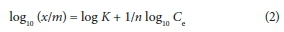
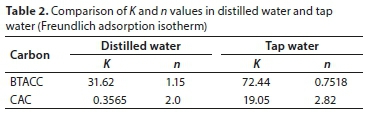
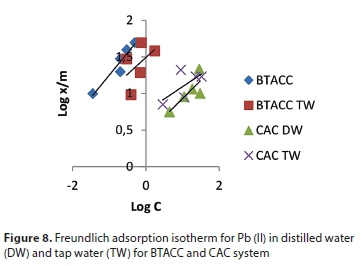
where Ce is the equilibrium concentration (mg∙L−1) and x/m is the amount adsorbed per unit weight of BTACC and CAC (mg∙g−1). Plots of log (x/m) vs. log Ce are linear for both BTACC and CAC (Fig. 8). The straight-line nature of the plots indicates that the process followed was of the Freundlich adsorption type; K and n values for both carbon sources were calculated from the intercepts and slopes, respectively, and are shown in Table 2. The values of 1 < n < 10 show favourable adsorption of Pb (II) on both BTACC and CAC (Guadalupe et al., 2008). The K values for BTACC and CAC are very much lower in distilled water than tap water because calcium and magnesium ions which are present in tap water compete for adsorption with lead.
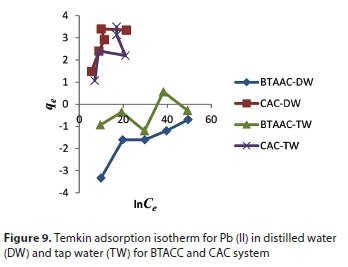
Temkin isotherm
Temkin considered the effects of some implied adsorbent-adsorbate interactions on the adsorption isotherms, suggesting that these interactions cause the heat of adsorption for all of the molecules in the layer to decrease linearly with the coverage. The Temkin isotherm has been applied in the following form (Mousavi et al., 2011):

where BT = RT/bT, T (K) is the absolute temperature, R is the universal gas constant (8.314 J∙mol−1 K−1), KT (L∙mg−1) is the equilibrium binding constant that corresponds to the maximum binding energy, bT is the variation in the adsorption energy (kJ∙mol−1), and BT is a Temkin constant related to the heat of adsorption (kJ∙mol−1). The Temkin constants can be derived by plotting qe versus lnCe. Ho et al. (1996) indicated that the typical range for the binding energy during ion exchange mechanism is 8-16 kJ∙mol−1. The heat of adsorption BT value for BTACC and CAC is 14.26 and 5.278 kJ∙mol−1, respectively. It is confirmed that BTACC has the binding energy within the limit 8-16 kJ∙mol−1 and it was found that the ion exchange mechanism took place while it further confirmed the ion exchange capacity value of BTACC. CAC, however, does not fall within the limit of binding energy (8-16 kJ∙mol−1).
Adsorption studies
In order to explain the adsorption mechanism and rate-controlling steps, the kinetic adsorption data were modelled using pseudo-first order and pseudo-second order kinetic equations.
Pseudo-first order kinetics
The pseudo-first order reaction equations of Lagergren (1898) have been widely used for the adsorption of liquid/solid systems on the basis of solid capacity. The linear form is generally expressed as:
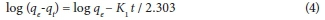
where qe (mg∙g−1) and qt (mg∙g−1) are the amount of adsorption at equilibrium at time t (min); k1 (min−1) is the rate constant of the pseudo-first-order adsorption process. The values of k1, R2 and qe at different concentrations were obtained by plotting log (qe − qt) versus t. The constants were determined experimentally by plotting of log (qe − qt) vs. t (Fig 10a, b).
Pseudo-second-order kinetics
The pseudo-second-order model obtained from Ho et al. (1999) is based on the assumption that the adsorption follows the second-order rate equation. The linear form can be written as:

where K2 is the rate constant of adsorption.
To evaluate the applicability of kinetic data relative deviation (P %) was calculated using the equation:
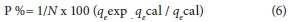
where qe (exp) and qe(cal) are the experimental and calculated value of Pb (II) adsorbed on the adsorbents, N is the number of measurements made. The lower the percentage deviation (P%), the better is the fit. When P (%) is less than 5, the fit is considered to be excellent (Ugurlu et al., 2009). The correlation coefficient (R2) for the pseudo-second-order model is much closer to unity. The calculated qe value was much closer to the experimental qe value. All kinetic parameters and correlation coefficients are listed in Table 5. Also the percentage relative deviation (P%) was found to be less than 5% in the case of pseudo-second-order. These values predict that the adsorption kinetics of Pb (II) ions onto the BTACC and CAC is mainly based on the pseudo-second-order equation. So the overall rate of Pb (II) ion adsorption may be controlled by the chemical process.
Adsorption mechanism
The pseudo-first-order and pseudo-second-order kinetic models were not able to explain the diffusion mechanism and also the rate-determining step for the adsorption of Pb (II) ions onto the BTACC and CAC. This is explained by the intra-particle diffusion model. For a solid-liquid sorption process, the solute transfer is usually characterized by either external mass transfer or intra-particle diffusion or both. During the adsorption of Pb (II) ions onto BTACC and CAC, the following three consecutive steps were involved (Acharya et al., 2009):
•The movement of adsorbate molecules from the bulk solution to the external surface of the adsorbent (film diffusion)
•Adsorbate molecules move to the interior part of the adsorbent particles (intra-particle diffusion)
•Sorption of the solute on the interior surface of the pores and capillary spaces of adsorbent (sorption)
The effects of contact time data were analysed by the Weber et al. (1963) intra-particle diffusion model to elucidate the diffusion mechanism; the model is expressed as:

where I (mg∙g−1) is the intercept and kd is the intra-particle diffusion rate constant (mg∙g−1∙min1/2), which can be evaluated from the slope of the linear plot of qt versus t1/2 as shown in Figs 10e and 10f. The dual nature of the curve was obtained due to the varying extent of adsorption in the initial and final stages of the adsorption experiment. This can be attributed to the fact that in the initial stages the sorption was due to the boundary layer diffusion effect whereas in the later stages (linear portion of the curve) it was due to the intra-particle diffusion effect. The intercept of the plot shows the boundary layer effect. The larger the intercept the greater will be the contribution of the surface sorption in the rate-controlling step. If the regression in the plot of qt versus t1/2 is linear and passes through the origin, then intra-particle diffusion is the sole rate-limiting step. However, in the present study, the plots were not linear and did not pass through the origin. The results show that intra-particle diffusion was not only the rate-limiting step, but also the rate-controlling step of adsorption.
Film and pore diffusion coefficient
For the adsorption of heavy metals on carbon surfaces, Michaelson et al., (1975) proposed film diffusion to be the rate-determining process, and the value of film diffusion co-efficient (Df) to be between 10−6 and 10-8 cm2∙s−1. If the pore diffusion were to be the rate-limiting factor, the pore diffusion co-efficient (Dp) should be in the range of 10−11-10−13 cm2∙s−1 (Senthil Kumar et al., 2009). Assuming a spherical geometry for the sorbents, the theoretical rate constant of the process can be correlated to the pore diffusion co-efficient in accordance with the expression:

where r0 is the radius of the sorbent expressed in cm2, Dp is the pore diffusion coefficient expressed in cm2∙s−1, Df the film diffusion co-efficient expressed in cm2∙s−1, cᵔ/c is the equilibrium loading factor of the sorbent and t1/2 is the half-life period expressed in seconds. The results for BTACC and CAC are given in Table 5.
CONCLUSION
The present investigation showed that carbon prepared from a novel waste material, BTACC, is able to remove of 98% Pb (II) ion from aqueous solution, whereas CAC removed only 52%. The presence of hydroxyl, carboxylic and sulphonic acid groups in BTACC was confirmed by FT-IR spectroscopy, and give ion exchange properties to the BTACC. The ion exchange value of BTACC was found to be 0.6024 molar equiv∙g−1. Compared with CAC, which has a very narrow range of pH 5, BTACC as a wider applicable pH range from 4-10 where the carbon is applied in wastewater treatment. The equilibrium data agreed well with the Langmuir adsorption value. The sorption kinetic pseudo-second-order model and intraparticle diffusion is not the sole rate-controlling factor. The value of the film diffusion co-efficient obtained for BTACC and CAC lies between 10−6 to 10-8 cm2∙s−1 for various initial concentrations of Pb (II) ions. Hence it is evident that Pb (II) removal follows film diffusion which is the rate-limiting factor. Therefore, it can be concluded that carbon derived from BTACC is very effective for the removal of Pb (II) from aqueous solution.
ACKNOWLEDGEMENTS
The authors are very thankful to Dr A Elango ME, Ph.D, Principal, Alagappa Chettiar College of Engineering and Technology, Karaikudi for providing necessary facilities and encouragement to complete this work. We also thank the management of CMS College of Engineering and Technology, Coimbatore for their constant support.
REFERENCES
ABBASZADEH S, RAFIDAH WAN ALWI S, WEBB C, GHASEMI N and MUHAMAD II (2016) Treatment of lead-contaminated water using activated carbon adsorbent from locally available papaya peel biowaste. J. Clean. Prod. 118 210-222. https://doi.org/10.1016/j.jclepro.2016.01.054 [ Links ]
ACHARYA J, SAHU JN, MOHANTY CR and MEIKAP BC (2009) Removal of lead (II) from wastewater by activated carbon developed from tamarind wood by zinc chloride activation. Chem. Eng. J. 149 249-262. https://doi.org/10.1016/j.cej.2008.10.029 [ Links ]
AHAMED M, VERMA S, KUMAR A and SIDDIQUI MKJ (2005) Environmental exposure to lead and its correlation with biochemical indices in children. Sci. Total Environ. 346 48-55. https://doi.org/10.1016/j.scitotenv.2004.12.019 [ Links ]
APHA, AWWA (1973) Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington D.C. [ Links ]
BABARINDE NAA, BABALOLA JO and SANNI RA (2006) Biosorption of lead ions from aqueous solution by maize leaf. Int. J. Phys. Sci. 1 23-26. [ Links ]
DEMIRBAS A (2008) Heavy metal adsorption onto agro-based waste materials: A review. J. Hazardous Mater. 157 220-229. https://doi.org/10.1016/j.jhazmat.2008.01.024 [ Links ]
GOEL J, KADIRVELU K, RAJAGOPAL C and KUMAR GARG V (2005) Removal of lead (II) by adsorption using treated granular activated carbon, batch and column studies. J. Hazardous Mater. 125 211-220. https://doi.org/10.1016/j.jhazmat.2005.05.032 [ Links ]
GROFFMAN A, PETERSON S and BROOKINS D (1992) Removing lead from wastewater using zeolite. Water Environ. Technol. 4 54-59. [ Links ]
GUADALUPE R, REYNEL-AVILA HE, BONILLA-PETRICIOLET A, CANO-RODRÍGUEZ I, VELASCO-SANTOS C and MARTÍNEZ-HERNÁNDEZ AL (2008) Recycling poultry feathers for Pb removal from wastewater: kinetic and equilibrium studies. Int. J. of Chem. Molec. Eng. 2 (11) 338-346. [ Links ]
HALL KR, EAGLETON LC, ACRIVOS A and VERMEULEN TH (1966) Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Fund. Ind. Eng. Chem. 5 212-223. https://doi.org/10.1021/i160018a011 [ Links ]
HO YS and MCKAY G (1999) Pseudo-second order model for sorption processes. ProcessBiochem. 34 451-465. https://doi.org/10.1016/s0032-9592(98)00112−5 [ Links ]
HO YS, WASE DAJ and FORSTER CF (1996) Removal of lead ions from aqueous solution using sphagnum moss peat as adsorbent. Water SA 22 219-224. [ Links ]
HUA M, ZHANG S, PAN B, ZHANG W, LU LV and ZHANG Q (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazardous Mater. 211 317- 331. https://doi.org/10.1016/j.jhazmat.2011.10.016 [ Links ]
INGLEZAKIS VJ, LOIZIDOU MD and GRIGOROPOULOU HP (2003) Ion exchange of Pb2+, Cu2+, Fe3+ and Cr3+ on natural clinoptilolite: selectivity determination and influence of acidity on metal uptake. J. Colloid Interf. Sci. 261 49-54. https://doi.org/10.1016/s0021-9797(02)00244-8 [ Links ]
JYOTIKUSUM A, SAHU JN, MOHANTY CR and MEIKAP BC (2009) Removal of lead (II) from wastewater by activated carbon developed from tamarind wood by zinc chloride activation. Chem. Eng. J. 149 249-262. https://doi.org/10.1016/j.cej.2008.10.029 [ Links ]
KADIRVELU K, THAMARAISELVI K and NAMASIVAYAM C (2001) Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol. 76 63-65. https://doi.org/10.1016/s0960-8524(00)00072-9 [ Links ]
LAGERGREN S (1898) About the theory of so-called adsorption of solute substances. Kungliga Svenska Vetenskapsakad Handlingar 24 1-39. [ Links ]
MICHELSON LD, GIDEON PG, PACE EG and KUTAL LH (1975) US Department of Industry, Office of Water Research and Technology, Bulletin 74. [ Links ]
MOUSAVI HZ and SEYEDI SR (2011) Nettle ash as a low cost adsorbent for the removal of nickel and cadmium from wastewater. Int. J. Environ. Sci. Technol. 8 (1) 195-202. https://doi.org/10.1007/bf03326209 [ Links ]
PATHANIA D, SHARMA S and SINGH P (2017) Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 10 1445-1451. https://doi.org/10.1016/j.arabjc.2013.04.021 [ Links ]
PEHLIVAN E, ALTUN T and PARLAYICI S (2008) Utilization of barley straws as biosorbents for Cu2+ and Pb2+ ions. J. Hazardous Mater. http://dx.doi.org/10.1016/j.jhazmat.08.115. [ Links ]
RAUNIO S and TAHATI H (2001) Glutamate and calcium uptake in astrocytes after acute lead exposure. Chemosphere 44 355-359. https://doi.org/10.1016/s0045−6535(00)00305-2 [ Links ]
RIAZA M, NADEEMA R, HANIFA MA, ANSARIC TM and REHMANA K (2009) Pb(II) biosorption from hazardous aqueous streams using Gossypium hirsutum (cotton) waste biomass. J. Hazardous Mater. 161 88-94. [ Links ]
SENTHIL KUMAR P and GAYATHRI R (2009) Adsorption of Pb2+ ions from aqueous solutions onto bael tree leaf powder. J. Eng. Sci. Technol. 4 381-399. [ Links ]
SRINIVASAN K and RAMADEVI A (2005) Removal of lead in aqueous medium by tamarind nut carbon. Ind. J. Environ. Protect. 25 420-428. [ Links ]
SURESH JEYAKUMAR RP and CHANDRASEKARAN V (2014) Adsorption of lead (II) ions by activated carbons prepared from marine green algae. Int. J. Ind. Chem. 5 (2) 1-10. https://doi.org/10.1007/s40090-014-0010-z [ Links ]
THARANITHARAN V and SRINIVASAN K (2009) Studies on the adsorption of Ni (ll) on to modified amberlite XAD-7HP resin. Ind. J. Environ. Protect. 29 (4) 294. [ Links ]
UGURLU M, KULA I, HAMDI KARAOGLU M and ARSLAN Y (2009) Removal of Ni(II) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation. Environ. Progress Sustainable Energ. 28 547-557. https://doi.org/10.1002/ep.10358 [ Links ]
WEBBER TN and CHAKRAVARTI RK (1974) Pore and solid diffusion models for fixed bed adsorbers. Am. Inst. Chem. Eng. 20 228-238. https://doi.org/10.1002/aic.690200204 [ Links ]
WEBER WJ and MORRIS JC (1963) Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 89 31-60. [ Links ]
WHO (World Health Organization) (1977) Environmental Health Criteria, 3, Lead. WHO, Geneva. [ Links ]
Received 11 June 2018
Accepted in revised form 4 June 2019
* Corresponding author, email: ramadeviramanujam61@gmail.com













