Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Enology and Viticulture
On-line version ISSN 2224-7904Print version ISSN 0253-939X
S. Afr. J. Enol. Vitic. vol.45 n.1 Stellenbosch 2024
https://doi.org/10.21548/45-1-6427
ARTICLES
Diversity of Xanthomonas citri pv. viticola Populations on Grapevines from Different Locations in India
S. BhosaleI; S. SahaII, *; N. PatilI; A. NazzarI
IMIT School of Bioengineering Sciences & Research, MIT ADT University, Loni Kalbhor, Maharashtra, India, 412 201
IIICAR-National Research Centre for Grapes, Plant Pathology, ICAR-National Research Centre for Grapes, P.B. No 3, Manjri Farm, Solapur Road, Pune, Maharashtra, India, 412 307
ABSTRACT
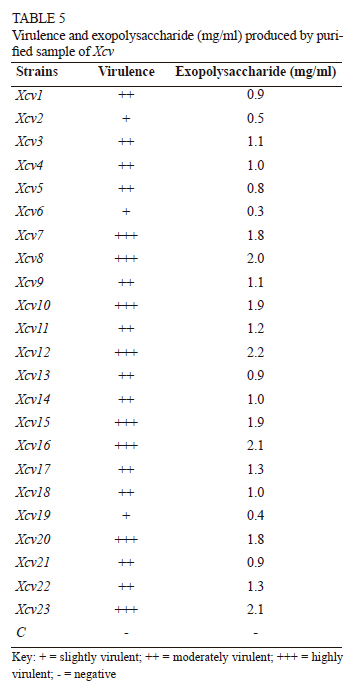
The bacterial leaf spot (BLS) disease caused by Xanthomonas citri pv. viticola (Xcv) is a menace to the production of grapes all over India. The maximum incidence of BLS has been reported in the Maharashtra, Karnataka, and Andhra Pradesh states of India. The symptoms are characterised by water-soaked, angular spots which later become necrotic on leaves. The present study was conducted to investigate the variability of 23 Xcv strains isolated from infected samples collected from Maharashtra, Karnataka, and Tamil Nadu in India. Samples were collected on the basis of the characteristic symptoms. The diversity of all the isolates was assessed phenotypically and genetically. Phenotypic characters included the morphology of colonies, pathogenicity, hypersensitivity, and biochemical tests. Genetic characterisation was assessed using 16S rRNA sequencing. The results exhibited diversity with respect to various phenotypic aspects, like colony size, colour and opacity, the methyl red reduction test, enzyme production, pathogenicity, and a hypersensitivity reaction. The 16S rRNA sequencing showed a distribution of pathogens into two main clusters, which were further divided into nine sub-clusters. The salient findings on Xcv diversity in India will be useful to identify and characterise resistant germplasms against the disease. The nonuniform variability obtained among the isolates suggests that geographical location, climatic factors and the varieties were the driving forces for the evolution of the phytopathogens.
Key words: Vitis vinifera, Xanthomonas citri pv. viticola, phenotypic diversity, genetic diversity
INTRODUCTION
The grape industry is one of the major fruit industries in India, and the total output of grape production reached up to 3 489 000 metric ton from an average area of 162 000 ha in the 2021/2022 production year (Anonymous, 2022). The major grape-growing states are Maharashtra and Karnataka, accounting for about 95% of India's total grape production. Other table grape-growing states include Tamil Nadu, Telangana, Andhra Pradesh, Mizoram, Punjab, Madhya Pradesh, Jammu and Kashmir, Nagaland, Haryana and Rajasthan (Anonymous, 2022). India is one of the biggest grape exporters in the world, with reported earnings of 313.69 million USD in 2022/2023 (Anonymous, 2022). However, grape-related diseases like powdery mildew, downy mildew, anthracnose, bacterial leaf spot and rust have affected the growth of the grape industry and caused huge economic losses. According to a report, India loses 8.23%, i.e. about 223 000 tons, of its grapes every year (Agricultural and Processed Food Products Export Development Authority [APEDA], 2021).
Bacterial leaf spot (BLS) is a disease of grapevine that occurs worldwide, especially during the months of August to September in warm and humid climates (Jones et al., 2014). Xanthomonas citri pv. viticola was reported to cause BLS of grapevine in Maharashtra, characterised by small, brown, angular, water-soaked lesions on the leaves, stems and fruit, leading to defoliation and direct fruit damage (Kamble et al., 2019). Severe infection may cause substantial damage to crops, with significant yield losses (Pernezny et al., 2003).
The causal bacteria are disseminated via various routes, like contaminated soil, leaves, berries, irrigation water and infected plant debris, which act as potential sources of inoculum (Gitaitis & Walcott, 2007). Initially, bacteria grow epiphytically and then enter the host through either stomata, hydathodes or wounds, spreading systemically to colonise the mesophyll parenchyma (Ryan et al., 2011). The distribution and prevalence of BLS-causing Xanthomonas species in India is relatively unknown. BLS was first reported in India by Nayudu (1972), but its causal agent was mentioned as Pseudomonas viticola. Later, Dye (1978) redefined its taxonomic position and named it X. campestris pv. viticola. Chand and Kishun (1990) also reported the epidemic occurrence of the disease in India and its extensive influence on yield loss. In 2018, Da Gama et al. suggested reclassification of X. campestris pv. viticola as X. citri pv. viticola.
BLS is primarily controlled by resistant lines, antibiotic treatment, and copper sprays (Roach et al., 2018). Due to reliance on the limited range of chemicals, resistance against copper and antibiotics was reported in the Xanthomonas population (Martin et al., 2004; Griffin et al., 2017). Hence, resistant lines, cultural interventions and biological control could be integrated for the successful management of the disease. The evolution of new Xanthomonas spp. and pathogenic strains over time has hindered the development and deployment of host resistance to manage bacterial spot disease in grape crops (Timilsina et al., 2016). Variation and variability are natural phenomena in plant pathogenic bacteria, and their identification is always a challenge. 16S rDNA sequencing is one of the most rapid and accurate methods for identifying disease-causing bacteria and understanding their epidemiology (Clarridge et al., 2004; Faniyan et al., 2023). The rRNA genes, such as 16S, 23S and 5S, are highly conserved at the genus and species levels, and thus are believed to be useful tools for grouping bacteria at the taxonomic level.
Although outbreaks of BLS have occurred in most commercial vine-growing regions of India, scanty information is available regarding the genetic diversity and distribution of the causal Xanthomonas species. The development of effective management approaches, particularly the selection of resistant plant material, relies upon the accurate identification of the pathogens and a thorough understanding of pathogen diversity and pathogenicity. This study describes the phenotypic and genetic variability of pathogenic Xanthomonas spp. associated with BLS in India.
MATERIALS AND METHODS
Sample collection
Infected leaf samples were collected from various grapevine varieties in 23 different locations across Maharashtra, Karnataka and Tamil Nadu (Table 1), according to the symptoms shown in Fig. 1. The leaves were collected in labelled polythene bags and stored at 4°C prior to use.

Isolation of Xanthomonas
The collected leaf samples were washed thoroughly under tap water and blot dried. Each leaf was segmented into pieces, 1 cm to 2 cm long, using a sterile scalpel. The pieces were dipped in sodium hypochlorite (1:100) (NaOCl) solution, followed by incubation for 30 sec, and subsequently treated with a 70% ethanol solution for another 30 sec. Finally, the leaf segments were washed twice with sterile distilled water, blot dried and placed on a sterile nutrient agar (NA) plate to be incubated at 37°C for 24 h. After 24 h, the observed colonies were selected and characterised morphologically (Costa et al., 2012). The selected colonies were sub-cultured and purified, following the method of Shah (2021) with minor modifications. Macroscopic features of the isolated bacterial colonies were assessed for the following criteria: colour, elevation, margin, opacity, consistency and surface of the colony. Microscopic features were determined through gram staining (Bartholomew & Mittwer, 1952).
Biochemical characterisation of purified bacterial isolates
The isolates were subjected to several biochemical tests -the potassium hydroxide (KOH) test, sugar utilisation test, catalase test, urease test, gelatine liquefaction test, indole production test, hydrogen cyanide (HCN) production test, ammonia production, starch hydrolysis test, lipase production test, methylene red reduction, nitrate reduction, oxidase test, citrate reduction, hydrogen sulphide (H2S) production, cellulase production and protease production (Schaad et al., 2001; Vashist et al., 2013).
Abiotic stress-tolerance assay
Estimation of salt tolerance
Fresh bacterial culture was inoculated in test tubes containing nutrient broth with different concentrations of sodium chloride (NaCl), viz. 2%, 4%, 6% and 8% (Ullah et al., 2018). Growth was observed after 24 h of incubation at 37°C, after which salt tolerance could be evaluated.
Estimation of temperature tolerance
Fresh bacterial culture was inoculated in test tubes containing nutrient broth. Growth was observed after 24 h of incubation at different temperatures, viz. 0°C, 4°C, 26°C and 30°C, after which temperature tolerance could be evaluated (Ullah et al., 2018).
Virulence test
For the assessment of the virulence of Xcv on a grape leaf, an inoculum suspension was prepared in sterile water. Pinprick and injection infiltration methods were used for inoculation. In the pin prick method, paper pins were used to bruise the leaves, and the inoculum (106 cfu/ml) of Xcv was applied. In the injection infiltration method, leaves of young seedlings were inoculated with 1 ml of inoculum using a hypodermic syringe. Control leaves (C) were inoculated with sterilised distilled water (Mazi et al., 2015).
Effect of different cultural conditions on the virulence of Xcv
Effect of age on the bacterium
Twenty-four hours of growth of the bacterial isolates on NA slants was used as a stock culture, and sub-culturing was done at one-day intervals in order to get five-, four-, three-, two- and one-day-old bacterial growth (Klement, 1963). Optical density of the bacterial suspension of each age level was adjusted to 0.5 and inoculated at the lower surface of the leaf using the syringe infiltration method. Symptoms were observed after 24 h of inoculation.
Effect of temperature
To study the effect of incubation temperature on virulence, each bacterial isolate was plated separately on NA and the plates were incubated at 25°C, 30°C and 35°C respectively. When the colonies emerged, one colony from each temperature treatment was sub-cultured, and the bacterial growth was suspended in sterile distilled water, centrifuged, and the optical density (OD) was adjusted to 0.5 (Klement, 1963). Isolates were inoculated at the lower surface of the leaf and symptoms were observed after 24 h.
Effect of pH
Nutrient agar plates were prepared with varying levels of pH, from 5.0 to 8.0 at intervals of 0.5. Fifty ml of the broth was dispensed in each of the 250 ml Erlenmeyer flasks and autoclaved at 121°C and 15 psi for 20 min. A loopful of 24 h growth of each isolate was suspended in 10 ml of sterile water. The flasks were then inoculated with 0.1 ml of the bacterial suspension prepared from the respective pH levels and incubated (Klement, 1963). Isolates were inoculated at the lower surface of the leaf and observed for lesions after 24 h of inoculation.
Exopolysaccharide (EPS) production
Cultures were grown in basal medium prepared by mixing dipotassium phosphate (K2HP04, 0.12%), potassium dihydrogen phosphate (KH2PO4, 0.08%), magnesium sulfate heptahydrate (MgSO4.7H2O, 0.02 %) and ammonium nitrate (NH4NO3, 0.5%) on a rotary shaker for seven days at 28°C to 30°C (Nayudu, 1972). Bacterial cells were removed by centrifugation at 15 000 rpm for 15 min. N-cetylpyridinium chloride (2.0 g per litre) was added to the supernatant for the precipitation of polysaccharide, which was quickened by the addition of one to two pellets of KOH. The brownish-white granular precipitate was removed by centrifugation and dissolved in 10% NaCI, and the polysaccharide were reprecipitated with two volumes of ethanol. This process was repeated thrice. The EPS produced was finally washed with ethanol, and dried before taking the final weight (Chowdhury et al., 1980).
Molecular identification
A single pure colony of the bacteria was inoculated in 100 ml of sterile nutrient broth (NB) and cultured on a rotatory incubator shaker at 120 rpm for 24 h at 28 ± 1°C. The genomic deoxyribonucleic acid (DNA) was extracted using the HiMedia Bacterial Genomic Kit according to the manufacturer's instructions, and quantified with a Nanophotometer® NP120 (IMPLEN ver. 2008).The universal primers 27F (5'-AGAGTTTGATCMTGGCTCAG-3') and 1492R (5'-GGTTACCTTGTTACGACTT-3') were used to amplify the 16S rDNA gene. Polymerase chain reaction amplifications were performed in 50 μ! reaction mixture including 2U Taq polymerase (Bangalore Genei, Bangalore, India), 5 μl of 10X Taq buffer, dNTP at 200 μM, 10 pmol of each primer (IDT, USA) and 25 ng of DNA template. Initial denaturation was carried out at 94°C for four minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, primer extension at 72°C for 1.5 minutes, and final extension at 72°C for 10 minutes. The sequencing of the resultant polymerase chain reaction (PCR) amplicons was outsourced (GeneMatrix, Pune, India). Using BioEdit Sequence Alignment Editor, the sequences were aligned to obtain consensus sequences, which were then compared to three sequences of Xcv species available at the National Centre for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov) using a BLAST search. MEGA 6.0 software was used to create the phylogenetic tree.
RESULTS
Isolation
Twenty-three isolates were obtained from the samples collected from various locations across India (Table 1). These isolates differed in morphological appearance, viz. colour, shape, size, margin, elevation, consistency and opacity.
Morphological characterisation of Xcv isolates
It was observed that all the Xcv colonies had the characteristic features, namely round and white in colour, convex, smooth and butyrous in texture. The size of all the isolates ranged from 1 mm to 3 mm in diameter. Eight isolates from the Palkhed (Xcv5), Karsul (Xcv9), Sarole (Xcv10), Belanki (Xcv11), Walwa (Xcv12), Kuchi (Xcv13), Narayangaon (Xcv16) and Mohal (Xcv17) locations were opaque in nature, while the rest were translucent (see Fig. 2 and Table 2).

Biochemical characterisation of Xcv isolates
All the isolates responded positively to the loop test by forming a thread when lifted gently. The loop formation provides confirmation of the Gram-negative bacteria to which all the isolates conformed. The isolates marked as Gram-negative from the loop test were further tested using Gram staining. Similar results were obtained twice, as all isolates retained a pinkish colour, thus confirming that they were Gram-negative.
All the isolates tested negative for H2S production, giving no black discoloration on lead acetate paper strips. It was observed that all the isolates had negative results for oxidase, urease and nitrate-reduction reactions, but were positive for the catalase test. All the isolates were able to ferment the tested sugars, viz. glucose, galactose, fructose, sucrose, dextrose and lactose. Isolates were neither able to utilise citrate as a sole source of carbon, nor did they show positive indole production. Isolates from eight locations, viz. Palkhed (Xcv5), Sarole (Xcv10), Walwa (Xcv12), Kuchi (Xcv13), Karoli (Xcv14), Pandharpur (Xcv18), Vijayapura (Xcv20) and Theni (Xcv21), were able to reduce methyl red reagent. It was observed that none of the isolates from Pune district were positive for the methyl red reduction test (Table 3a).
It was noted that eight isolates, from the Bhuvana (Xcv1), Satana (Xcv8), Walwa (Xcv 12), Kuchi (Xcv13), Vijayapura (Xcv19 and Xcv20) and Theni (Xcv21 and Xcv21) regions, were unable to produce protease, lipase or gelatinase enzymes. Protease enzyme was produced by the isolates from Bhuvana (Xcv2), Verkheda (Xcv6), Balanki (Xcv11), Karoli (Xcv14), Narayangaon (Xcv16), Mohal (Xcv17), Pandharpur (Xcv18) and Theni (Xcv22), whereas lipase was secreted by isolates from Verkheda (Xcv6), Sarole (Xcv10), Pandharpur (Xcv18) and Theni (Xcv22). Ten isolates -from Sompur (Xcv2), Thengoda (Xcv4), Palkhed (Xcv5), Verkheda (Xcv7), Karsul (Xcv9), Sarole (Xcv10), Belanki (XcvH), Narayangaon (Xcv15), Pandharpur (Xcv18) and Theni (Xcv22) - were able to hydrolyse gelatine. Amylase and cellulase enzymes were produced by all the isolates from the different locations (Table 3b).
Abiotic stresses
None of the isolates were able to tolerate temperature stress. The maximum growth of Xcv took place at 26°C, and no growth was observed at 0°C, 4°C and -30°C. Tolerance to different salt concentrations were tested, and all the isolates were able to tolerate salt concentration of up to 4%, as no growth was observed in broth with a higher salt concentration (Table 4).
Virulence test
The infected grape leaves reacted positively to all isolates within 24 h to 72 h. Mild chlorosis to brown necrosis was observed around the injection point (Fig. 2). As per virulence reaction, the pathogen was categorised as strongly virulent, moderately virulent and mildly virulent. Isolates from eight locations, viz. Verkheda (Xcv7), Satana (Xcv8), Sarole (Xcv10), Walwa (Xcv12), Narayangaon (Xcv15, Xcv16), Vijayapura (Xcv20) and Theni (Xcv23), were considered to be highly virulent, as they manifested symptoms within 24 h of inoculation, whereas 12 isolates, from Bhuvana (Xcv1), Thengoda (Xcv3, Xcv4), Palkhed (Xcv5), Karsul (Xcv9), Belanki (Xcv11), Kuchi (Xcv13), Karoli (Xcv14), Mohal (Xcv17), Pandharpur (Xcv18) and Theni (Xcv21 and Xcv22), were regarded as moderately virulent, as the symptoms were seen within 48 h. After 72 h of inoculation, symptoms appeared in three of the isolates from Sompur (Xcv2), Verkheda (Xcv6) and Vijayapura (Xcv19) locations, and these can be categorised as slightly virulent. It is clear from the data that Nashik and Sangli had the highest number of highly virulent strains, whereas strains from Pune, Theni and Solapur were of moderate virulence. No symptoms were recorded in the controls inoculated with distilled water (Table 5).
Two inoculation methods, viz. pin prick and syringe infiltration, were used, and they gave positive responses on the host plants. However, the syringe infiltration method gave more prominent symptoms compared to the pin prick method. The syringe infiltration method therefore was used for the further studies.
Effect of different cultural conditions on the virulence of Xcv
Effect of age of the bacterium
The virulence of the bacterial isolates causing BLS was influenced by the age and origin of the bacterial strains. Isolates from Sompur (Xcv2), Thengoda (Xcv3), Palkhed (Xcv5), Verkheda (Xcv7), Satana (Xcv8), Walwa (Xcv12), Karoli (Xcv 14), Narayangaon (Xcv15, Xcv16), Mohal (Xcv17), Vijayapura (Xcv20) and Theni (Xcv22, Xcv23) manifested the highest virulence in three- and four-day-old cultures, whereas isolates from Bhuvana (Xcv1), Thengoda (Xcv4), Verkheda (Xcv6), Karsul (Xcv9), Sarole (Xcv10), Belanki (Xcv11), Kuchi (Xcv13), Pandharpur (Xcv18), Vijayapura (Xcv19) and Theni (Xcv21) showed the highest virulence in two- and three-day-old cultures. Three-day-old cultures of all isolates manifested the maximum virulence compared to the other age levels. Five- and one-day-old cultures were the least virulent. The interaction between the bacterial isolates and their age in relation to virulence was significant.
Effect of temperature
Incubation temperature also influenced the virulence of the bacterial isolates. The pathogen was more virulent when it was incubated at 30°C. However, the virulence of the pathogen was reduced when incubated at 25°C and 35°°C, although these did not show a significant difference.
Effect of pH
The pH levels, ranging from 5.0 to 8.0, influenced the virulence of the causal bacterial isolates. All the isolates were significantly virulent at all the tested pH levels, with no direct relationship manifested between the pH levels and the virulence of the bacterial isolates.
Exopolysaccharide production
All the strains were able to produce a significant amount of exopolysaccharides (EPS), ranging from 0.3 mg/ml to 2.2 mg/ml (Table 5). Highly virulent strains of Xcv were able to produce larger amounts of EPS compared to the others. Isolates Xcv7 (1.8 mg/ml), Xcv8 (2.0 mg/ml), Xcv10 (1.9 mg/ml), Xcv12 (2.2 mg/ml), Xcv15 (1.9 mg/ml), Xcv16 (2.1 mg/ml), Xcv20 (1.8 mg/ml) and Xcv23 (2.1 mg.ml), from Verkheda, Satana, Sarole, Walwa, Narayangaon, Vijayapura and Theni respectively, produced the maximum amount of EPS, ranging from 1.8 mg/ml to 2.2 mg/ml. Isolates from Bhuvana (Xcv1), Thengoda (Xcv3, Xcv4), Palkhed (Xcv5), Karsul (Xcv9), Belanki (Xcv11), Kuchi (Xcv13), Karoli (Xcv14), Mohal (Xcv17), Pandharpur (Xcv18) and Theni (Xcv21 and Xcv22) produced moderate amounts of EPS, ranging from 0.8 mg/ml to 1.3 mg/ml. The smallest amounts of EPS were produced by Xcv2 (0.5 mg/ml), Xcv6 (0.3 mg/ ml) and Xcv19 (0.4 mg/ml) from Sompur, Verkheda and Vijayapura respectively (Table 5).
Molecular characterisation and cluster analysis
Polymerase chain reaction products produced a single band of approximately 1 500 base pairs (bp). 16S rDNA sequencing of these PCR-amplified products showed diversity in the Xanthomonas community. All the isolates had more than 97% sequence similarity with the Xanthomonas citri pv. viticola strain in a BLAST search. Sequences from seven Xanthomonas isolates were obtained from NCBI and subjected to multiple alignments. A dendrogram depicting the estimated phylogenetic relationships was constructed by the neighbour-joining clustering method (Fig. 3). This dendrogram was based on comparisons of all the available 16S rDNA sequence data for the genus Xanthomonas citri pv. viticola species. The sequence obtained was submitted to GeneBank and an accession number was procured (Table 6). The cluster analysis also clearly discriminated Xanthomonas citri pv. viticola causing bacterial leafspot of grapes from other Xanthomonas species infecting different hosts (Fig. 3).
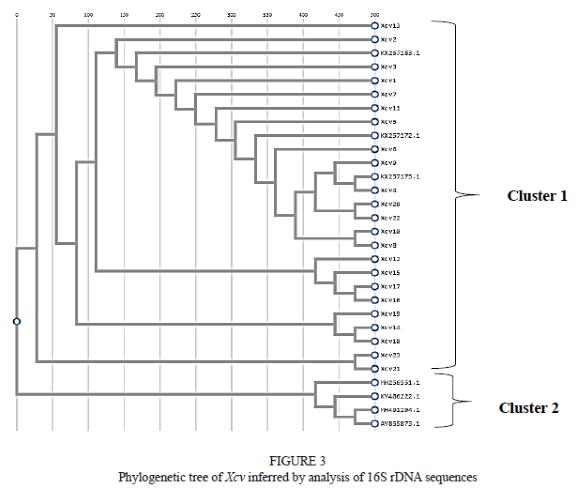
The dendrogram constructed from the pooled data had two main clusters (Fig. 3) - cluster I and cluster II. A clear distinction could be observed among the isolated Xcv and other Xanthomonas strains, as all the Xcv were grouped under cluster I, whereas cluster II consisted ofXanthomonas strains, viz. Xanthomonas albilineans (MH491194.1), Xanthomonas sacchari (KY486222.1), Xanthomonas campestris (MH256551.1) and Xanthomonas translucens pv. graminis (AY855873.1). Cluster I was further divided into two sub-clusters. Isolates from Karoli (Xcv14), Pandharpur (Xcv18) and Vijayapur (Xcv19) were found to be closely related to each other. Isolates from the Walwa (Xcv12), Narayangaon (Xcv15 and Xcv16) and Mohal (Xcv17) regions were grouped in one sub-cluster, whereas isolates from Theni (Xcv21 and Xcv23) fell into another. All three Xcv isolates retrieved from the NCBI were closely related to the remaining isolates, as all were grouped in the same sub-cluster.
DISCUSSION
The pathogenic and genetic diversity of the Xcv strains associated with BLS disease in India were analysed through pathogenicity, standard bacteriological tests and 16S rRNA sequence analysis. The results suggest that the Xcv populations investigated in the present study were composed of pathologically and genetically diverse strains.
The present study supports the previous findings relating to the occurrence of bacterial leaf spot in India and the characterisation of the causal bacterium, Xcv (Kamble et al., 2019), and confirms that the disease is widespread in the grape-growing regions of India. Symptoms of the BLS, e.g. water-soaked, angular small spots on the lower surface of the leaves, were observed in all the visited fields. Similar symptoms of BLS have been described by Araujo and Robbs (2000) and Nascimento and Mariano (2004). Necrosis, yellow spots and cankerous lesions on the leaves and shoots are characteristic symptoms caused by any pathogen belonging to the genus Xanthomonas (Bradbury, 1970). The symptoms observed on the grapevines were homologous with the previous studies (Bradbury, 1970; Kamble et al., 2019).
Bacteria of the genus Xanthomonas are straight, Gram-negative rods, typically with yellow pigmentation and a polar flagellum, strictly aerobic chemoorganotrophs, and mostly phytopathogenic. As observed, the colony morphology of Xcv revealed white-coloured colonies. In contrast to the present finding, a yellow-coloured colony of bacterial leaf spot pathogen of grapes was reported by Jambenal et al. (2011), which hints at phenotypic diversity. Hence, yellow colonies produce the pigment Xanthomonadin (Goel et al., 2001), while white or albino colonies do not produce this pigment due to acquired mutations in the Xanthomonadin biosynthesis gene cluster, such as frameshift mutation, deletion and insertion, cause them to lose their pigmentation (Midha & Patil, 2014). Hence there is phenotypic diversity. Similar findings have been made in the case of other albino strains, Xanthomonas campestris pv. mangiferaeindicae and Xanthomonas axonopodis pv. ricini (Gama et al., 2011). The size and shape of colonies were small to medium, convex and mucoid. The staining reaction was observed under microscope, and the bacteria were found to be Gram negative. The bacterial colonies identified were 1 mm to 3 mm in diameter, and Gram-negative cells were observed under the microscope. These observations regarding colony morphology in the present study correlate with reports by Breed et al. (1989) and Kamble et al. (2019). The results support those of a previous study (Arshiya et al., 2014) in which morphological, biochemical and pathogenicity tests were performed to identify and characterise the strains of Xanthomonas causing bacterial disease. The biochemical test results analysed in the present study confirm the pathogen.
In the present study, variations were observed with respect to the morphological and biochemical characterisation. All Xcv were able to utilise carbon in all the tested forms. This finding is in contrast with previous results reported by Young and Triggs (1994), who found that pathovars of P. syringae pv. maculicola were heterogeneous in their carbon source utilisation. Giri et al. (2011) reported that, amongst 16 strains of Xanthomonas axonopodis pv. punicae, only five could hydrolyse the starch and almost all the strains produced acid from different carbon sources. They further added that 13 strains had produced H2S gas. The results reflect the biochemical variation exhibited by the strains of X. axonopodis pv. punicae from different geographical locations (Tables 3a and 3b). Several researchers have reported variations in biochemical tests within the different pathovars (Wiebe et al., 1993; Clerc et al. 1998; Zhao et al., 2008).
Exopolysaccharide production is an important virulence determinant in numerous plant pathogenic bacteria, including Xanthomonas spp., Erwinia amylovora and Pseudomonas syringae, during infection by pathogens (Kemp et al., 2004). The present study shows that all the pathogenic Xcv were able to produce a significant amount of EPS. It was also seen that EPS production was directly proportional to the virulence, which supports the previous findings of Guo et al. (2015) and Nguyen et al. (2016). These authors reported that X. oryzae pv. oryzae and X. citri subsp. citri employed multiple virulence factors to promote their pathogenicity on rice and citrus respectively, including EPS production. Exopolysaccharide production helps the pathogen to grow and spread in plants by protecting the organism against toxic compounds like superoxide radicals, hydrogen peroxide (H2O2), and high or low pH produced by the hosts in defence reactions (Yu et al., 2016; Bae et al., 2018).
Here, we present a systematic approach to generating and validating diversity among species/supraspecies levels using the 16S rRNA gene, which was also proved by Escapa et al. (2020). Previous studies on membrane protein analysis, fatty acid profiling and 16s rRNA analysis showed a high similarity among several strains or pathovars ofXanthomonas (Ndongo et al., 2018). Comparisons of sequence variation in the 16S rRNA region among 23 strains of Xcv from different locations showed more than 97% similarity. In addition, partial 16S rRNA sequences were identical among the strains of Xcv studied, and the current findings support the study of Kamble et al. (2019). There is limited information describing the genetic diversity of Xcv, compared to the closely related pathovars of Xcc.
There was a correlation between the groups and the geographical origin of the strains, e.g. from Maharashtra, Karnataka and Tamil Nadu (Fig. 3). Alberts et al. (2002) state that it is plausible that the two groups may signify distinct, recent introductions of the pathogen, even though the origin of the distribution is not clear. Previous reports about Xanthomonas pv. manihotis have shown that there is divergence among the strains that were recently introduced to Africa (Verdier et al., 1993). Linkages between groups, indicated by rep-PCR, and geographical origin have been reported for Xanthomonas strains pathogenic to other plants. Scortichini et al. (2001) reported the existence of genetic diversity in a worldwide collection of X. axonopodis pv. juglandis isolated from walnuts, and showed that the genetics of the pathogen was unique to each walnut cultivation area. Massomo et al. (2003) observed variation among X. campestris pv. campestris strains isolated from Brassica sp. collected in fields in Tanzania, and linked fingerprint patterns to specific geographical areas. They noted that adaptability might be the basis for this phenomenon. Likewise, Mkandawire et al. (2004) revealed that X. campestris pv. phaseoli strains isolated from the common bean of Africa comprised three genotypes - two unique to East Africa and the other associated with strains collected from the New World. Selection for a specialised niche can affect genome organisation and the distribution of repetitive sequences in the bacterium genome, resulting in fingerprints unique to a specific pathovar or strain (Louws et al., 1994). Yap et al. (2004) reported genetic diversity in E. carotovora subsp. carotovora strains isolated from the same region in the same season from the same host species. It would be interesting to study the Xanthomonas citri pv. viticola adaptation when different areas or climatic factors are involved.
The genetic diversity of Xcv was found to be based on the different geographical locations in India. The reasons for significant differences are yet to be investigated. However, several factors, such as the migration of individuals, sampling strategies and lumping of strains from different locations, could be possible mechanisms for such variations. Although Xcv populations appeared to be highly diverse, one possible explanation for the current observations might be horizontal gene transfer (HGT) and recombination, which probably occurred within the vines (Filip & Skuza, 2021; Ghaly et al., 2024). It has been reported that HGT is the predominant force leading to similarities between the genomes of X. citri pv. fuscans and X. phaseoli pv. phaseoli. HGT was the main reason for the evolution of Xanthomonas strains with the common bean as host (Chen et al., 2018). Further studies on Xanthomonas citri pv. viticola are necessary to clarify the transfer mechanism. Timilsina et al. (2019) say that the core genes reveal the extent, source and mechanisms of recombination events that shaped the current population and genomic structure of X. perforans in Florida.
CONCLUSION
This study provides new insight into the phenotypic and genotypic diversity and evolution of Xanthomonas citri pv viticola strains - the causal agent of bacterial leaf spot in grapevines - from Maharashtra, Karnataka and Tamil Nadu in India. Although the sample size was limited, the results indicate diversity in the population structure. Future studies are necessary to identify the factors responsible for the resistance in Xcv. Finally, this work provides an excellent basis for further exploration of the specific interaction between the Xcv strain and grapevines that will help to implement various preventive strategies. Furthermore, it can help to minimise the losses due to BLS through the development of a PCR-based diagnostic method for early detection of the pathogen.
LITERATURE CITED
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K. & Walter, P., 2002 (4th ed). Molecular biology of the cell. Introduction to pathogens. Garland Science, New York. https://www.ncbi.nlm.nih.gov/books/NBK26917/ [ Links ]
Agricultural and Processed Food Products Export Development Authority (APEDA), 2021. Grapes. https://apeda.gov.in/apedawebsite/SubHead_Products/Grapes.htm [ Links ]
Anonymous, 2022. NRCG, Pune, 2022. https://nrcgrapes.icar.gov.in/for%20farmers/Annexure%205_RMP_Grapes_202223_24.08.2022.pdf [ Links ]
Araújo, J.S.P. & Robbs, C.F., 2000. Sintomatologia, patogenicidade e controle do cancro bacteriano da videira (Xanthomonas campestris pv. viticola) no Brasil. Agronomia 34, 83-86. [ Links ]
Arshiya, M., Suryawanshi, A., More, D. & Baig, M.M.V., 2014. Repetitive PCR based detection of genetic diversity in Xanthomonas axonopodis pv. citri strains. J. App. Biol. Biotechnol. 2(1), 17-22. [ Links ]
Bae, N., Park, H.J., Park, H., Kim, M. & Han, S.W., 2018. Deciphering the functions of the outer membrane porin OprBXo involved in virulence, motility, exopolysaccharide production, biofilm formation and stress tolerance in Xanthomonas oryzae pv. oryzae. Mol. Plant Pathol. 19(12), 2527-2542. [ Links ]
Bartholomew, J.W. & Mittwer, T., 1952. The gram stain. Bacteriol. Rev. 16(1), 1-29. [ Links ]
Bradbury, J.F., 1970. Xanthomonas oryzicola. [Descriptions of fungi and bacteria]. Descriptions of Fungi and Bacteria 24, Sheet 240. [ Links ]
Breed, R.S., Murry, E.G.D. & Smith, N.R., 1989. Bergey's manual of systemic bacteriology. S.T. Williums. [ Links ]
Chand, R. & Kishun, R., 1990. Outbreak of grapevine bacterial canker disease in India. Vitis 29(3), 183-188. [ Links ]
Chen, N.W.G., Serres-Giardi, L., Ruh, M., Briand, M., Bonneau, S., Darrasse, A., Barbe, V., Gagnevin, L., Koebnik, R. & Jacques, M.A., 2018. Horizontal gene transfer plays a major role in the pathological convergence of Xanthomonas lineages on common bean. BMC Genom. 19(1), 606. https://doi.org/10.1186/s12864-018-4975-4 [ Links ]
Chowdhury, H.D. & Verma, J.P., 1980. Exopolysaccharide production by pathogenic and nonpathogenic bacteria associated with leaves of cotton. Indian Phytopathol. 33(2), 304-307. [ Links ]
Clarridge, J.E., 2004. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 17(4), 840-862. https://doi.org/10.1128/CMR.17.4.840-862.2004 [ Links ]
Clerc, A., Manceau, C. & Nesme, X., 1998. Comparison of randomly amplified polymorphic DNA with amplified fragment length polymorphism to assess genetic diversity and genetic relatedness within genospecies III of Pseudomonas syringae. Appl. Environ. Microbiol. 64, 1180-1187. [ Links ]
Costa, L.E.D.O., Queiroz, M.V.D., Borges, A.C., Moraes, C.A.D. & Araújo, E.F.D., 2012. Isolation and characterization of endophytic bacteria isolated from the leaves of the common bean (Phaseolus vulgaris). Braz. J. Microbiol. 43, 1562-1575. [ Links ]
Da Gama, M.A.S., De Mariano, R.L.R., Da Silva Júnior, W.J., De Farias, A.R.G., Barbosa, M.A.G., Ferreira, M.Á., Costa Júnior, C.R.L., Santos, L.A. & De Souza, E.B., 2018. Taxonomic repositioning of Xanthomonas campestris pv. viticola (Nayudu 1972) dye 1978 as Xanthomonas citri pv. viticola (Nayudu 1972) dye 1978 comb. nov. and emendation of the description of Xanthomonas citri pv. anacardii to include pigmented isolates pathogenic to cashew plant. Phytopathology 108, 1143-1153. [ Links ]
Escapa, F.I., Huang, Y., Chen, T., Lin, M., Kokaras, A., Dewhirst, F.E. & Lemon, K.P., 2020. Construction of habitat-specific training sets to achieve species-level assignment in 16S rRNA gene datasets. Microbiome 8, 65. https://doi.org/10.1186/s40168-020-00841-w [ Links ]
Faniyan, O., Akpe, V. & Cock, I.E., 2023. Analyzing bacterial species from different environments using direct 16S rRNA gene sequencing methods. Pharmacogn. Commun. 13(1), 24-33. [ Links ]
Filip, E. & Skuza, L., 2021. Horizontal gene transfer involving chloroplasts. Int. J Mol. Sci. 22(9), 4484. [ Links ]
Gama, M.A.S., Mariano, R.L.R., Viana, F.M.P., Ferreira, M.A.S.V. & Souza, E.B., 2011. Polyphasic characterization of pigmented strains of Xanthomonas pathogenic to cashew trees. Plant Dis. 95(7), 793-802. [ Links ]
Ghaly, T.M., Gillings, M.R., Rajabal, V., Paulsen, I.T. & Tetu, S.G., 2024. Horizontal gene transfer in plant microbiomes: Integrons as hotspots for cross-species gene exchange. Front. Microbiol. 15, 1338026. [ Links ]
Giri, M.S., Prasanthi, S., Kulkarni, S., Benagi, V.I. & Hegde, Y.R., 2011. Biochemical and molecular variability among Xanthomonas axonopodis pv. punicae strains, the pathogen of pomegranate bacterial blight. Indian Phytopathol. 64(1). https://epubs.icar.org.in/index.php/IPPJ/article/view/6001 [ Links ]
Gitaitis, R. & Walcott, R., 2007. The epidemiology and management of seedborne bacterial diseases. Annu. Rev. Phytopathol. 45, 371-397. [ Links ]
Goel, A.K., Rajagopal, L. & Sonti, R.V., 2001. Pigment and virulence deficiencies associated with mutations in the aroE gene of Xanthomonas oryzae pv. oryzae. Appl. Environ. Microbiol. 67(1), 245-250. https://doi.org/10.1128/AEM.67.1.245-250.2001 [ Links ]
Griffin, K., Gambley, C., Brown, P. & Li, Y., 2017. Copper-tolerance in Pseudomonas syringae pv. tomato and Xanthomonas spp. and the control of diseases associated with these pathogens in tomato and pepper. A systematic literature review. Crop Prot. 96, 144-150. [ Links ]
Guo, W., Zou, L.F., Cai, L.L. & Chen, G.Y., 2015. Glucose-6-phosphate dehydrogenase is required for extracellular polysaccharide production, cell motility and the full virulence of Xanthomonas oryzae pv. oryzicola. Microb. Pathog. 78, 87-94. [ Links ]
Jambenal, S., Ravikumar, M.R. & Hiremani, N., 2011. Basic studies on Xanthomonas campestris pv. viticola causing bacterial leaf spot of grape and evaluated in-vitro efficacy of different chemicals and bioagents against its growth. Int. J. Plant Prot. 4(2), 397-401. [ Links ]
Jones, J.B., Zitter, T.A., Momol, M.T. & Miller, S.A., 2014 (2nd ed). Compendium of tomato diseases and pests. APS Press, St. Paul, MN. [ Links ]
Kamble, A.K., Sawan, S.D., Sawant, I.S., Ghule, S.B., Patii, A.C. & Saha, S., 2019. Characterization of Xanthomonas campestris pv. viticola causing bacterial leaf spot of grapes in Maharashtra, India. J. Environ. Biol. 40(6), 1145-1150. [ Links ]
Kemp, B.P., Horne, J., Bryant, A. & Cooper, R.M., 2004. Xanthomonas axonopodis pv. manihotis gumD gene is essential for EPS production and pathogenicity and enhances epiphytic survival on cassava (Manihot esculenta). Physiol. Mol. Plant Pathol. 64(4), 209-218. [ Links ]
Klement, Z., 1963. Rapid detection of the pathogenicity of phytopathogenic Pseudomonas. Nature 199, 299-293. [ Links ]
Louws, F.J., Fulbright, D.W., Stephens, C.T. & De Bruijn, F.J., 1994. Specific genomic fingerprints of phytopathogenic Xanthomonas and Pseudomonas pathovars and strains generated with repetitive sequences and PCR. Appl. Environ. Microbiol. 60, 2286-2295. [ Links ]
Martin, H.L., Hamilton, V.A. & Kopittke, R.A., 2004. Copper tolerance in Australian populations of Xanthomonas campestris pv. vesicatoria contributes to poor field control of bacterial spot of pepper. Plant Dis. 88(9), 921-924. [ Links ]
Massomo, S.M.S., Nielsen, H., Mabagala, R.B., Mansfeld-Giese, K., Hockenhull, J. & Mortensen, C.N., 2003. Identification and characterisation of Xanthomonas campestris pv. campestris strains from Tanzania by pathogenicity tests, Biolog, rep-PCR and fatty acid methyl ester analysis. Eur. J. Plant Pathol. 109, 775-789. [ Links ]
Midha, S. & Patil, P.B., 2014. Genomic insights into the evolutionary origin of Xanthomonas axonopodis pv. citri and its ecological relatives. Appl. Environ. Microbiol. 80(20), 6266-6279. [ Links ]
Mkandawire, A.B., Mabagala, R.B., Guzmán, P., Gepts, P. & Gilbertson, R.L., 2004. Genetic diversity and pathogenic variation of common blight bacteria (Xanthomonas campestris pv. phaseoli and X. campestris pv. phaseoli var. fuscans) suggests pathogen coevolution with the common bean. Phytopathology 94(6), 593-603. [ Links ]
Nascimento, A.R.P. & Mariano, R.D.L.R., 2004. Bacterial canker of grapevine: Etiology, epidemiology and control strategies. Ciência Rural 34(1), 301-307. [ Links ]
Nayudu, M.V., 1972. Pseudomonas vitícola sp. nov., incitant of a new bacterial disease of grapevine. J. Phytopathol. 73, 183-186. https://doi.org/10.1111/j.1439-0434.1972.tb02539.x [ Links ]
Ndongo, S., Beye, M., Dubourg, G., Nguyen, T.T., Couderc, C., Fabrizio, D.P., Fournier, P.E., Raoult, D. & Angelakis, E., 2018. Genome analysis and description of Xanthomonas massiliensis sp. nov., a new species isolated from human faeces. New Microb. New Infec. 26, 63-72. [ Links ]
Nguyen, M.P., Park, J., Cho, M.H. & Lee, S.W., 2016. Role of DetR in defence is critical for virulence of Xanthomonas oryzae pv. oryzae. Mol. Plant Pathol. 17(4), 601-613. [ Links ]
Pernezny, K., Roberts, P.D., Murphy, J.F. & Goldberg, N.P. (Eds.), 2003. Compendium of pepper diseases. APS Press, St. Paul, MN. [ Links ]
Roach, R., Mann, R., Gambley, C.G., Shivas, R.G. & Rodoni, B., 2018. Identification oí Xanthomonas species associated with bacterial leaf spot of tomato, capsicum and chilli crops in eastern Australia. Eur. J. Plant Pathol. 150, 595-608. [ Links ]
Ryan, R.P., Vorhölter, F.J., Potnis, N., Jones, J.B., Van Sluys, M.A., Bogdanove, A.J. & Dow, J.M., 2011. Pathogenomics of Xanthomonas: Understanding bacterium-plant interactions. Nat. Rev. Microbiol. 9(5), 344-355. [ Links ]
Schaad, N.W., Jones, J.B. & Chun, W., 2001 (3rd ed). Laboratory guide for the identification of plant pathogenic bacteria. St. Paul, MN, APS Press. [ Links ]
Scortichini, M., Marchesi, U. & Di Prospero, P., 2001. Genetic diversity of Xanthomonas arboricola pv. juglandis (synonyms: X. campestris pv. juglandis; X. juglandis pv. juglandis) strains from different geographical areas shown by repetitive polymerase chain reaction genomic fingerprinting. J. Phytopathol. 149, 325-332 [ Links ]
Shah, D., Khan, M.S., Aziz, S., Ali, H. & Pecoraro, L., 2021. Molecular and biochemical characterization, antimicrobial activity, stress tolerance, and plant growth-promoting effect of endophytic bacteria isolated from wheat varieties. Microorganisms 10(1), 21. [ Links ]
Thapa, S. P., Park, H. R., Lim, C. K. & Hur, J., (2011). H. Phylogeny of the Korean Erwinia Species as Determined by Comparison of 16S rDNA Sequences. Journal of Agricultural, Life and Environmental Sciences, 23(4), 62-69. [ Links ]
Timilsina, S., Abrahamian, P., Potnis, N., Minsavage, G., White, F., Staskawicz, B.J., Jones, J.B., Vallad, G.E. & Goss, E.M., 2016. Analysis of sequenced genomes of Xanthomonas perforans identifies candidate targets for resistance breeding in tomato. Phytopathology 106(10), 1097-1104. [ Links ]
Timilsina, S., Pereira-Martin, J.A., Minsavage, G.V., Iruegas-Bocardo, F., Abrahamian, P., Potnis, N., Kolaczkowski, B., Vallad, G.E., Goss, E.M. & Jones, J.B., 2019. Multiple recombination events drive the current genetic structure of Xanthomonas perforans in Florida. Front. Microbiol. 10, 448. https://doi.org/10.3389/fmicb.2019.00448 [ Links ]
Ullah, S., Bramley, H., Daetwyler, H., He, S., Mahmood, T., Thistlethwaite, R. & Trethowan, R., 2018. Genetic contribution of emmer wheat (Triticum dicoccon Schrank) to heat tolerance of bread wheat. Front. Plant Sci. 9, 1529. [ Links ]
Vashist, H., Sharma, D. & Gupta, A., 2013. A review on commonly used biochemical test for bacteria. Innovare J. Life Sci. 1(1), 1-7. [ Links ]
Verdier, V., Dongo, P. & Boher, B., 1993. Assessment of genetic diversity among strains of Xanthomonas campestris pv. manihotis. Microbiology 139(11), 2591-2601. [ Links ]
Wiebe, W.L. & Campbell, R.N., 1993. Characterization of Pseudomonas syringae pv. maulicola and comparison with P. s. pv. tomato. Plant Dis. 77, 414-419. [ Links ]
Yap, M.N., Barak, J.D. & Charkowski, A.O., 2004. Genomic diversity of Erwinia carotovora subsp. carotovora and its correlation with virulence. Appl. Environ. Microbiol. 70(5), 3013. [ Links ]
Young, J.M. & Triggs, C.M., 1994. Evaluation of determinative tests for pathovars of Pseudomonas syringae van Hall 1902. J. Appl. Bacteriol. 77(2), 195-207. [ Links ]
Yu, Y.H., Hu, Z., Dong, H.J., Ma, J.C. & Wang, H.H., 2016. Xanthomonas campestris FabH is required for branched-chain fatty acid and DSF-family quorum sensing signal biosynthesis. Sci. Rep. 6, 32811. https://doi.org/10.1038/srep32811 [ Links ]
Zhao, H.P., Wang, L., Ren, J.R., Li, Z., Li, M. & Gao, H.W., 2008. Isolation and characterization of phenanthrene-degrading strains Sphingomonas sp. ZP1 and Tistrella sp. ZP5. J. Hazard. Mater. 152(3), 1293-1300. [ Links ]
Submitted for publication: March 2024
Accepted for publication: May 2024
* Corresponding author: sujoyta@gmail.com
Acknowledgements: The authors would like to thank the Director of the ICAR-National Research Centre for Grapes, and the Director and Head of School of the MIT School of Bioengineering Sciences and Research, for their support during the study.
Data availability: The datasets presented in this study can be found in online repositories. The name of the repository is NCBI, and all the accession numbers are provided as supplementary data













