Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
https://doi.org/10.17159/2254-8854/2023/a15585
RESEARCH ARTICLE
A checklist of the dacine fruit flies (Diptera, Tephritidae, Dacinae) of Mozambique
Marc De MeyerI; Luis BotaII; Beatriz DanielIII; Mirene MussumbeIII; Myriam VandenboschI; Laura CanhangaIII; Elias CambulaIV; Mervyn W. MansellV; Domingos CugalaIII; Massimiliano VirgilioI
IRoyal Museum for Central Africa, Invertebrates Section & JEMU, Tervuren, Belgium
IIProvincial Directorate of Agriculture and Fisheries, National Fruit fly Laboratory, Chimoio, Mozambique
IIIEduardo Mondlane University, Faculty of Agronomy and Forest Engineering, Maputo, Mozambique
IVMinistry of Agriculture and Food Security, Department of Plant Protection, Maputo, Mozambique
VDepartment of Zoology and Entomology, University of Pretoria, Pretoria, South Africa
ABSTRACT
Here we present a checklist of all records of dacine fruit flies (Diptera: Tephritidae: Dacinae) from Mozambique, based on verified specimen records in natural history collections and literature records. In total, 57 Dacinae species are recorded from Mozambique, of which only one is considered endemic. This relatively low species diversity compared to other African countries appears to be related to incomplete sampling. For each species the localities from which it was recorded are given (including geocoordinates), or a general distribution is provided. The checklist is discussed briefly in terms of species richness, endemism and geographic distribution.
Keywords: distribution, diversity, surveys
INTRODUCTION
Fruit flies (Diptera, Tephritidae) are a very diverse group of Diptera, comprising more than 5 000 species worldwide (EFSA 2020), and approximately 1 000 species known from the Afrotropical region (Norrbom et al. 1999). The family is characterised by a diverse larval developmental biology mainly infesting the seed-bearing organs of a wide variety of host plants. This can take place either in the flower heads or in the fruits (the fleshy part or the actual seeds) (Drew and Yuval 1999). Because of this diversity in host range they can be used as indicator species for biodiversity (e.g. Copeland et al. 2005). A number of frugivorous species are, however, also of major economic significance because of the damage they cause on cultivated fruits including several that are categorised as vegetables (mainly Cucurbitaceae and Solanaceae such as cucumber, pumpkin, tomato, eggplant, etc.). Dacine fruit flies (Tephritidae: Dacinae) mainly belong to the frugivorous group, and thus comprise a number of pest species. Representatives of the tribe Gastrozonini who are grass infesters (in Asia in particular of bamboo. African records are from the subfamily Panicoideae, see Hancock 1999) are an exception among the Dacinae.
Losses for particular crops can run up to 40-50% and into the millions (see Ekesi et al. 2016 for examples) and the total loss across the entire African continent is estimated at USD 2 billion annually (Korir et al. 2015). Because of this economic importance of frugivorous species, the group has received a lot of scientific interest in Africa. However, for Mozambique this interest has been relatively limited prior to 2000. The oldest dated specimen known from Mozambique is a female of Ceratitis capitata (in the United States National Museum, Washington DC, U.S.A.) collected in Maputo in 1903. But von Röder (1885) and Karsch (1887) described a number of species based on specimens collected in 'Delagoa Bay' (i.e. Baia de Maputo), hence must have been collected prior to 1885. Further records are rare with the exception of a number of collecting efforts in 1928 (mainly at Umbeluzi River and Marracuene) reported by Munro (1984). Further material was collected by Skinner and McGough in 1949 within the framework of United States Department of Agriculture (USDA) funded surveys in Africa in search of natural enemies of C. capitata, and by Usher and Stuckenberg in 1957 in the area of Luabo and Mt Gorongosa. The presence of Bactrocera dorsalis (under the junior synonym Bactrocera invadens) in Mozambique in 2007 (Correia et al. 2008) triggered several surveying activities in the country funded by USDA/APHIS (Animal and Plant Health Inspection Service)-Pretoria office and conducted by mixed teams of USDA, EMU and RMCA. This yielded extensive collections from different provinces within the country not just for Bactrocera dorsalis but also for a number of other fruit fly species. The intensive sampling and subsequent data have been presented by Cugala et al. (2016), including information on their relative abundance. More recently, the invasion and spread of Zeugodacus cucurbitae has caused further concern and support has been given through several projects funded by the World Trade Organization's Standards and Trade Development Facility and the Belgian Development Cooperation (see https://fruitflies.africamuseum.be/activities/projects).
Published lists for Mozambique are nevertheless incomplete. For example the catalogue of the Afrotropical region (Cogan and Munro 1980) only lists nine species specifically occurring in Mozambique (and an additional 5 listed as 'widespread' in Africa). Garcia and Bandeira (2011) provide a general list of known Tephritidae from Mozambique, predominantly based on literature references. They report 59 species for the family, of which 35 belong to the Dacinae. In addition, prior to 2000, several species were known from a single or very limited number of localities and their distribution in the country poorly known. It was, therefore, considered important to have an up-to-date checklist of dacine fruit flies with their known distribution listed, so that it can be used as a reference publication for future research.
MATERIAL AND METHODS
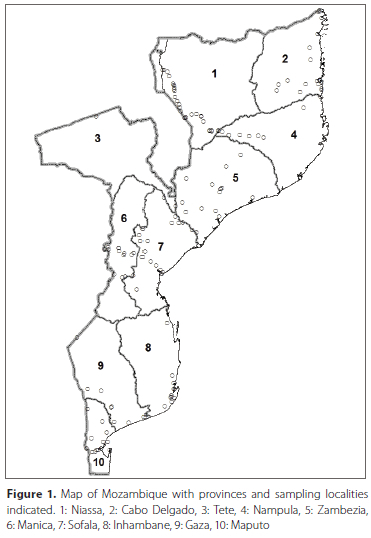
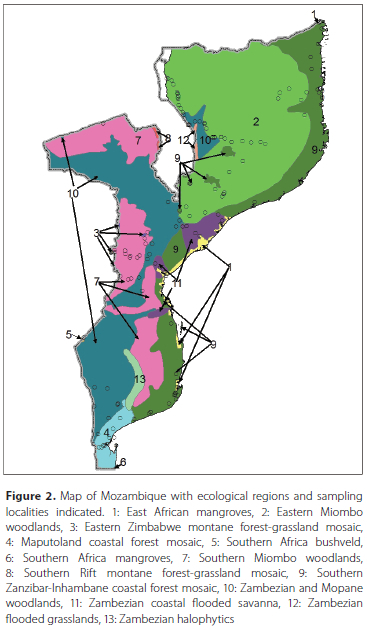
The checklist is based upon a specimen database (in Microsoft Access©) for Afrotropical dacine (Tephritidae, Dacinae) fruit flies, maintained by the RMCA. This database includes information for all specimens housed in the main natural history collections worldwide and of which the identification could be confirmed by re-examination. It also includes literature records that are deemed reliable. In total, it comprises 600 block records (i.e. a record for one or several specimens with identical data regarding locality, date of capture and collector) from Mozambique, representing more than 14 000 specimens. Records originate from all parts and provinces of Mozambique except for Tete province which has been poorly sampled (Figure 1). Mozambique has several ecological regions (Burgess et al. 2004) but a large part of the country is dominated by miombo woodlands and the majority of the localities where material has been collected correspond with this vegetation type. In addition, material has also been collected to some extent in the coastal forest mosaics. Other ecological regions like flooded grasslands or savannas, mangroves and montane grasslands are under sampled (Figure 2). Extensive references and list of collections that were examined, can be consulted at a dedicated website (True fruit flies (Diptera, Tephritidae) of the afrotropical regions - http://projects.bebif.be/fruitfly/index.html). Identifications are based on the respective papers listed below under the short treatment per genus, as well as the multi-entry lucid key developed by Virgilio et al. (2014) (which is also available online, see https://fruitflykeys.africamuseum.be).
For each taxon, the known list of localities in Mozambique is given. All these localities are included in Appendix 1 with their geo-coordinates where applicable and annotations for doubtful or unknown locations. If more than 30 localities within Mozambique are known for a particular taxon, it is indicated as 'widespread'. Additional information is given between parentheses "( )" for a particular location if deemed relevant. If the actual location could not be traced but only a related larger or a nearby geographical entity, the latter is given with the former between square brackets "[ ]". In addition to the distribution in Mozambique, the wider geographic distribution in Africa is also listed. If a particular taxon is known from more than 10 countries, a general description (like 'widespread' or 'widespread in eastern and southern Africa') is given. Furthermore we indicate for each species whether it is, based on the currently known host range as entered in the above mentioned database, either a nonpest, minor economic pest or major economic pest species. The listing is ordered by genus, and for each genus is preceded by a short description on the general biology and the availability of taxonomic revision and/or identification key. These are replicates of descriptions recently presented in an annotated checklist for the dacines of Tanzania (De Meyer et al. 2023). Comments are added under particular taxa when additional information on the record or identity is relevant.
In Appendix 1, all localities are listed with indication of the province (if this could be retrieved) and georeferences. The source of the georeferences varies and is either taken from ad verbatim indication on the collecting event or publication, or a posteriori sourced from various geographical reference sources and gazetteers.
LIST OF SPECIES
CERATITIDINI
Capparimyia Bezzi, 1920
The genus Capparimyia is a predominantly Afrotropical group, comprising eight species. All known and confirmed host records belong to the caper family (Capparaceae). Its biology is unusual in that larvae are reported both from flower buds and from fruits. A taxonomic revision and identification key are presented by De Meyer & Freidberg (2005).
Capparimyia aristata De Meyer & Freidberg
Mozambique: Mphingwe Also recorded from Malawi.
Status: non-pest (host unknown)
Carpophthoromyia Austen, 1910
The genus Carpophthoromyia comprises 17 species, all restricted to the Afrotropical Region. Known host plants belong to the genus Drypetes (Euphorbiaceae). An identification key and taxonomic revision are presented by De Meyer (2006).
Carpophthoromyia dimidiata Bezzi, 1924
Mozambique: Baia de Maputo, West of Beira [Siluwe Hills], Mphingwe, Pungwe River Valley [Guéngère], Tembe-Tembe, and Vanduzi
Widespread in southern and eastern Africa (Kenya, Malawi, South Africa, Tanzania, and Zimbabwe).
Status: non-pest
Comment: The record for Mozambique of C. vittata (Fabricius, 1794) in Norrbom et al. (1999) (and taken over in Garcia and Bandeira 2011) is probably based on a misidentification as all material studied by De Meyer (2006) from Mozambique belongs to C. dimidiata, and both species show a disjunct distribution. For an extensive discussion on taxonomic confusion between C. amoena Enderlein, 1920, C. dimidiata and C. vittata and their respective geographic ranges, see De Meyer (2006).
Ceratitis MacLeay, 1829
The genus Ceratitis is a very speciose (about 100 described species) Afrotropical genus. One species, C. capitata has been introduced into other parts of the world and is considered one of the economically most important pest species. Host range within the genus is variable with some species being restricted to a particular plant host genus while others are very polyphagous. De Meyer et al. (2002) presented a host plant list for all described species. A comprehensive multi-entry key for the genus is presented by Virgilio et al. (2014) and available online. See https://fruitflykeys.africamuseum.be
Ceratitis capitata (Wiedemann, 1824)
Mozambique: widespread
Widespread in Africa; introduced in other continents.
Status: major economic pest
Ceratitis cosyra (Walker, 1849)
Mozambique: widespread
Widespread in Africa.
Status: major economic pest
Ceratitis discussa Munro, 1935
Mozambique: Buzi River pontoon (near Chimanimani Mountains)
Also recorded from Benin, Kenya, Malawi, South Africa, Tanzania, and Zimbabwe.
Status: non-pest (identification unconfirmed for single record from Citrus (De Meyer et al., 2002)).
Ceratitis ditissima (Munro, 1938)
Mozambique: Luabo, and Rotanda (Mussapa River Forest) Widespread in Africa.
Status: minor economic pest (mainly of Citrus)
Ceratitis edwardsi (Munro, 1957)
Mozambique: Rotanda (Mussapa River Forest) Widespread in Africa.
Status: non-pest
Ceratitis marriotti Munro, 1933
Mozambique: Gorongosa Mountain Also recorded from Democratic Republic of Congo (Kivu region), Kenya, South Africa, and Uganda.
Status: non-pest
Ceratitis millicentae De Meyer & Copeland 2005
Mozambique: Boane, Chokwe, Jangamo, Lua-Lua, Maputo, Mecufi, Metuge, Mitucue, Mphingwe, Muchara, Ngoma, Vanduzi, and Zandamela
Also recorded from Eswatini, Kenya, and South Africa.
Status: non-pest
Ceratitis pedestris (Bezzi, 1924)
Mozambique: Maputo, Mitucue, and Mphingwe Widespread in Africa.
Status: minor economic pest (few records from tomato, main host Strychnos spp.)
Ceratitis punctata (Wiedemann, 1824)
Mozambique: unknown locality (one male specimen in Hendel Collection at Naturhistorisches Museum Wien, Austria). Garcia and Bandeira (2011) report this species from Umbeluzi. Widespread in Africa.
Status: minor economic pest
Comment: Because of possible confusion with C. millicentae (see De Meyer & Copeland 2005), the presence of this species in Mozambique needs reconfirmation.
Ceratitis quilicii De Meyer, Mwatawala & Virgilio, 2016
Mozambique: Maputo, Namaacha, and Quinta das Abelhas Also recorded from Botswana, Eswatini, Kenya, Malawi, Mauritius, Réunion, South Africa, Tanzania, and Zimbabwe.
Status: major economic pest
Comment: Prior to 2016, representatives of this species were listed under C. rosa. Re-examination of all available material shows that C. quilicii is limited to the southernmost part of the country.
Ceratitis quinaria (Bezzi, 1918)
Mozambique: Nanhupo, and Vanduzi
Widespread in Africa.
Status: minor economic pest (most records from mango, guava, and peach).
Ceratitis rosa Karsch, 1887
Mozambique: widespread
Also recorded from Kenya, Malawi, South Africa, and Tanzania.
Status: major economic pest
Ceratitis rubivora Coquillett, 1901
Mozambique: Malica, and Vanduzi
Also recorded from Burundi, Cameroon, Kenya, Malawi, South Africa, Tanzania, Uganda, and Zimbabwe.
Status: minor economic pest (of Rubus spp.).
Perilampsis Bezzi, 1920
Perilampsis comprises 17 species occurring solely in different parts of the Afrotropical region. All known hosts belong to Loranthaceae and the fruit fly larvae are known to attack the seeds. A taxonomic revision and identification key are provided by De Meyer (2009).
Perilampsis curta Munro, 1938
Mozambique: Boane, Maputo, and Masiena
Also recorded from Botswana, Eswatini, Kenya, South Africa, and Tanzania.
Comment: An earlier record by De Meyer (2009) of material from Maputo and identified as Perilampsis miratrix Munro, 1939 was considered erroneous after molecular identification and belongs to P. curta. The presence of P. miratrix in Mozambique by Garcia & Bandeira (2011) (misspelled as miriatrix), is thus not substantiated.
Status: non-pest
Trirhithrum Bezzi, 1918
The genus Trirhithrum is endemic to the Afrotropical region and includes about 40 described species. Host range is variable with some species considered pest species of coffee. An identification key is provided by White et al. (2003).
Trirhithrum albomaculatum (von Röder, 1885)
Mozambique: Baia de Maputo
Also recorded from Kenya, South Africa, Tanzania, Uganda, and Zimbabwe.
Status: non-pest
Trirhithrum bimaculatum (von Röder, 1885)
Mozambique: Baia de Maputo
Also recorded from Equatorial Guinea.
Status: non-pest (host unknown)
Trirhithrum nigerrimum (Bezzi, 1913)
Mozambique: Mphingwe
Widespread in Africa.
Status: minor economic pest (of coffee)
Trirhithrum nitidum (von Röder, 1885)
Mozambique: Baia de Maputo, and Maxixe Also recorded from South Africa.
Status: non-pest
DACINI
Genus Bactrocera Macquart, 1835
A very speciose genus (> 500 species) mainly found in the Oriental and Oceanian regions (Doorenweerd et al. 2018). Several of the representatives are considered agricultural pests and of major economic significance (White and Elson-Harris 1994). The African diversity is contrastingly rather limited with 13 species known from the Afrotropical region including three invasive species introduced from Asia: Bactrocera dorsalis, B. latifrons (Hendel, 1915) and B. zonata (Saunders, 1842). A key for the Afrotropical species is provided by White (2006) but not including B. latifrons.
Bactrocera biguttula (Bezzi, 1922)
Mozambique: Catuane, and Maputo
Also recorded from Kenya, and South Africa.
Status: non-pest (recorded from olive but needs confirmation (White, 2006)).
Bactrocera dorsalis (Hendel, 1912)
Mozambique: widespread
Widespread in Africa.
Status: major economic pest
Dacus Fabricius, 1805
The genus Dacus is an Old World genus comprising about 270 species worldwide with a predominance in the Afrotropical region (Doorenweerd et al. 2018). The host range is confined to three families: Apocynaceae, Cucurbitaceae, and Passifloraceae. The vast majority appear to be oligophagous attacking plants of one particular host family and host specificity is to some extent corroborated by monophyletic groupings within the genus (Virgilio et al. 2009; Starkie et al. 2022). However, the current subgeneric classification does not reflect this and is in need of revision. An identification key for the Afrotropical species is provided by White (2006) while some additional species were described afterwards by White and Goodger (2009) and De Meyer et al. (2013).
Dacus africanus Adams, 1905
Mozambique: Chupanga, Cuamba, Goonda, Gorongosa (town), Gorongosa Mountain, Mitucue, Morrumbala, Mphingwe, Nharuchonga, and Zero
Also recorded from South Africa, Zambia, and Zimbabwe.
Status: non-pest (host unknown)
Dacus amphoratus (Munro, 1984)
Mozambique: Marracuene, and Umbeluzi River Also recorded from Kenya, and Zimbabwe.
Status: non-pest (pest unknown)
Dacus binotatus Loew, 1862 Mozambique: Umbeluzi River
Also recorded from Democratic Republic of Congo, Kenya, Lesotho, Namibia, Nigeria, South Africa, and Zimbabwe.
Status: non-pest
Dacus bivittatus (Bigot, 1858)
Mozambique: widespread Widespread in Africa.
Status: major economic pest
Dacus brevis Coquillett, 1901
Mozambique: Umbeluzi River
Also recorded from Angola, Kenya, Lesotho, South Africa, Tanzania, Uganda, and Zimbabwe.
Status: non-pest
Dacus brevistriga Walker, 1861
Mozambique: Maputo
Also recorded from South Africa, and Tanzania.
Status: non-pest
Dacus ceropegiae (Munro, 1984)
Mozambique: Bandeze
Also recorded from Ethiopia, Kenya, Nigeria, Tanzania, and Uganda.
Status: non-pest
Dacus chapini Curran, 1927
Mozambique: Maputo
Also recorded from Cameroon, Democratic Republic of Congo, Kenya, Nigeria, Rwanda, Tanzania, and Uganda.
Status: non-pest (host unknown)
Dacus chiwira Hancock, 1985
Mozambique: Alua, Bive, Caia-Chupanga road (crossing), Cuamba, Goonda, Metuge, Mieze, Mitucue, Mphingwe, and Napaha
Also recorded from Ethiopia, Malawi, Tanzania, and Zimbabwe.
Status: non-pest
Dacus ciliatus (Loew, 1892)
Mozambique: Boane, Chande, near Chemba [Nova Choupanga], Cuamba, Fevia, Inhaca Island, Lichinga (Agricultural Research Station), Luabo, Magene, Magude, Maputo, Marracuene, Marromeu, Mugene, Namaacha, and Nharuchonga Widespread in Africa.
Status: major economic pest
Dacus durbanensis Munro, 1935
Mozambique: Caia-Chupanga road (crossing), Gorongosa (town), Mitucue, and Mphingwe
Also recorded from Kenya, Malawi, South Africa, Tanzania, and Zimbabwe.
Status: non-pest
Dacus eclipsis (Bezzi, 1924)
Mozambique: Boane, and Inhambane
Also recorded from South Africa, and Zimbabwe.
Status: non-pest
Dacus eminus Munro, 1939
Mozambique: Boane, Dombe, and Mphingwe
Also recorded from Angola, Namibia, South Africa, Zambia, and Zimbabwe.
Status: non-pest
Dacus famona Hancock, 1985
Mozambique: Bive, Cuamba, Mandimba (border post office), Manhica, Mitucue, Mutuale, and Nanhupo Also recorded from Botswana, Malawi, South Africa, Tanzania, Zambia, and Zimbabwe.
Status: non-pest (host unknown)
Dacus ficicola Bezzi, 1915
Mozambique: Umbeluzi River
Also recorded from Angola, Democratic Republic of Congo, Kenya, Lesotho, Rwanda, Somalia, South Africa, Zambia, and Zimbabwe.
Status: non-pest
Dacus frontalis Becker, 1922
Mozambique: Boane, and Namaacha Widespread in Africa.
Status: minor economic pest
Dacus fuscatus Wiedemann, 1819
Mozambique: unknown locality (type material of junior synonym Dacus bistrigatus Loew, 1852. Syntype in Zoological Museum of Berlin but only thorax and midlegs remaining; whereabouts of other syntype material unknown. (See White 2006) Also recorded from Angola, Botswana, Lesotho, South Africa, Tanzania, and Zimbabwe.
Comments: this species is listed as Dacus bistrigatus by Garcia and Bandeira (2011).
Status: non-pest
Dacus fuscinervis (Malloch, 1932)
Mozambique: Maputo
Also recorded from Kenya, South Africa, and Tanzania.
Status: non-pest (host unknown)
Dacus hamatus Bezzi, 1917
Mozambique: Chimoio, and Maputo Widespread in Africa.
Status: non-pest
Dacus humeralis (Bezzi, 1915)
Mozambique: Mphingwe, and Namaacha Widespread in Africa.
Status: non-pest
Dacus kariba Hancock, 1985
Mozambique: Gorongosa Mountain Also recorded from South Africa, Zambia, and Zimbabwe.
Status: non-pest (host unknown)
Dacus mulgens Munro, 1932
Mozambique: Baia de Maputo
Also recorded from Lesotho, and South Africa.
Status: non-pest
Dacus ostiofaciens Munro, 1932
Mozambique: Maputo
Also recorded from Kenya, and South Africa.
Status: non-pest
Dacus pallidilatus Munro, 1948
Mozambique: Alua, Chiure, Cuamba, Dombe, and Mphingwe Also recorded from Tanzania, and Zimbabwe.
Status: non-pest
Dacus pamelae (Munro, 1984)
Mozambique: Luabo
Only known from Mozambique.
Status: non-pest (host unknown)
Dacus pergulariae Munro, 1938
Mozambique: unknown locality (one male specimen in collection Naturhistorisches Museum Wien without further details)
Also recorded from Ethiopia, Kenya, South Africa, and Tanzania.
Status: non-pest.
Dacus plagiatus Collart, 1935
Mozambique: Maputo, and Umbeluzi River
Also recorded from Democratic Republic of Congo, Kenya, Nigeria, South Africa, Tanzania, and Zimbabwe.
Status: non-pest (host unknown)
Dacus punctatifrons Karsch, 1887
Mozambique: Boane, Chiure, Chokwe, Cuamba, Dombe, Gorongosa (town), Lichinga (6 km S), Macomia, Mandimba, Manhica, Maputo, Mecufi, Metuge, Mieze, Mitucue, Mocimboa da Praia, Mocuba, Mphingwe, Mutuale, Muxungue, Napaha, Naucheche, Pemba, and Vanduzi Widespread in Africa.
Status: major economic pest
Dacus purpurifrons Bezzi, 1924
Mozambique: Umbeluzi River
Also recorded from Democratic Republic of Congo, South Africa, and Zimbabwe.
Status: non-pest (host unknown).
Dacus siliqualactis Munro, 1939
Mozambique: Covane Community Lodge (Canhane, Massingir) Widespread in Africa.
Status: non-pest
Dacus umbeluzinus Munro, 1984
Mozambique: Umbeluzi River
Also recorded from Benin, Burkina Faso, Kenya, Tanzania, and Zimbabwe.
Status: non-pest
Dacus vertebratus Bezzi, 1908
Mozambique: Boane, near Chemba [Nova Choupanga], Maputo, Pungwe River Valley [Guéngère], and Rikatla Widespread in Africa.
Status: major economic pest
Genus Zeugodacus Hendel, 1927
A speciose genus comprising close to 200 species, all restricted to the Oriental and Oceanian regions (Doorenweerd et al. 2018). One species, the melon fly Zeugodacus cucurbitae, has been introduced to other parts of the world including the African continent as well as some to the islands group in the western Indian Ocean (White 2006).
Zeugodacus cucurbitae (Coquillett, 1899)
Mozambique: Present in northern and central parts of the country: Cabo Delgado, Nampula, Niassa, Zambezia, Tete, Manica and Sofala provinces. (cf. discussion below) Widespread in Africa.
Status: major economic pest
GASTROZONINI
Bistrispinaria Speiser, 1913
The genus Bistrispinaria comprises four endemic species. Known host records belong to Poaceae (Copeland 2007). An identification key was provided by Hancock (1999).
Bistrispinaria magniceps (Bezzi, 1918)
Mozambique: Amatongas
Also recorded from Burundi, Democratic Republic of Congo, Kenya, Malawi, Sudan, Tanzania, and Uganda.
Status: non-pest
Clinotaenia Bezzi, 1920
The genus Clinotaenia comprises six endemic species. There are no known host records but it is assumed that they attack Poaceae as is the case with other gastrozonines. Hancock (1999) provides an identification key for five species (C. angusticeps (Bezzi, 1923) was later transferred to this genus by De Meyer (2006)).
Clinotaenia superba (Bezzi, 1918)
Mozambique: Serra Chiperone (east of), and near Vanduzi [Belasse]
Also recorded from Malawi, and Tanzania.
Status: non-pest
DISCUSSION
In total, 57 Dacinae species are recorded from Mozambique. This is about 14% of all dacine species recorded from the Afrotropical region. Compared to some of the neighbouring countries like Tanzania (117 species known) or South Africa (104 species), this is a relatively low number. It does not appear to be due to less varied ecosystems being presented in Mozambique but rather to incomplete sampling throughout all regions of the country. Compared to other neighbouring countries where there has been limited sampling in recent decades as well, such as Malawi (59 species), Zimbabwe (68 species) or Zambia (27 species), the number is comparable.
Of all species known from Mozambique, only one appears to be endemic: Dacus pamelae described from Luabo at the lower Zambezi River and collected there by P and B Stuckenberg in 1957. The species has not been reported since. Regarding distribution patterns within the country, the information is also patchy because of the limited distribution data for several species and thus any conclusions or patterns are preliminary. Many species' distribution fits in a more general pattern from southern to eastern Africa. This is, for example, reflected in Carpophthoromyia dimidiata, Ceratitis millicentae, C. quilicii, C. rosa, Perilampsis curta, and several Dacus species. For some the presence in Mozambique is the northernmost distribution record and is even restricted to the southern provinces, like observed in Trirhithrum nitidum, and Dacus mulgens.
One species, Dacus longistylus Wiedemann, 1830 is reported to occur in Mozambique according to Norrbom et al. (1999) (reference repeated in Garcia & Bandeira 2011). However, no material belonging to this species could be studied by the authors. It is also not listed as occurring in Mozambique in the revision by White (2006). Dacus longistylus has a wide distribution in Africa but largely from the West (Senegal) eastwards to the Arabian Peninsula. The southernmost record is from central Tanzania (see De Meyer et al. 2023. However, as the host for this species is Calotropis procera (Apocynaceae) and this plant occurs in parts of Mozambique, the presence of the fruit fly is not unlikely. We prefer, however, to await the actual confirmation based on examined material from the country before including it in the species list. The presence of Perilampsis miratrix is based on a misidentification by De Meyer (2009) and actually refers to P. curta while the presence of Ceratitis punctata requires confirmation because of possible confusion with C. millicentae (cf. above under comments in species list).
Two species found in Mozambique are invasives from Asia: Bactrocera dorsalis and Zeugodacus cucurbitae. The former was first recorded from the Kenyan coast in 2003 by Lux et al. 2003 (as member of the Bactocera dorsalis group; later described as Bactrocera invadens by Drew et al. 2005, but this is generally considered as junior synonym of B. dorsalis). The first occurrence for Mozambique was reported by Correia et al. (2008) from Cuamba in Niassa Province. Its rapid spread through Mozambique has been well documented by Cugala et al. (2016) with the species spreading southwards throughout the whole country over five years (2007-2012). It is now found throughout the whole of Sub-Saharan Africa with the exception of the more southern provinces in South Africa (Manrakhan et al. 2015). Zeugodacus cucurbitae was already present in eastern Africa (Kenya and Tanzania) since the 1930s. For a long time its distribution appeared to be restricted to some areas in eastern Africa but since 2000 it has been reported from several other countries throughout the African continent and it is now widely distributed from Senegal eastwards to Kenya and southwards to Malawi and Mozambique (De Meyer et al. 2015). In Mozambique it was first reported in 2013 from the northernmost districts Mocimboa da Praia and Palma of Cabo Delgado Province (Cugala et al. 2014). In 2016 the species was considered to be still restricted to this area although in higher abundance (Cugala et al. 2016). Recent surveys, however, have indicated that the species is now spreading southwards as well, having been detected in several sampling sites in the northern (Cabo Delgado, Nampula and Niassa provinces) and Central (Tete, Manica, Sofala and Zambezia provinces) regions, where it was not reported previously. There has been no detection of Z. cucurbitae at the trapping sites in the southern provinces of Inhambane, Gaza and Maputo.
Of all species reported, 39 were already known to occur in Mozambique before 2000. However of these 29 were known from a single location in Mozambique only. Several of these records were restricted to a few sampling sites, in particular the capital Maputo (or Delagoa Bay/Baia de Maputo as a larger area), and the adjacent Umbeluzi River area, both in the southernmost part of the country. Since 2000, 18 additional species were found and the geographic spread for a number of species is now better documented. However, in general the main distribution and range of the majority of the species remains poorly known and more intensive surveys are required in order to get a more complete image of the dacine fruit fly diversity and occurrence in the country.
ACKNOWLEDGEMENTS
The authors acknowledge the support and funding of the following institutions and organisations: the Belgian Development Cooperation through consecutive framework agreements with the Royal Museum for Central Africa (projects NSS fruit flies, AGROVEG, DISPEST), the Standards and Trade Development Facility (STDF) of the World Trade Organisation (project FFF), and the USDA-APHIS Pretoria office for support of initial surveys for Bactrocera dorsalis occurrence in Mozambique. We would like to thank the reviewers for their valuable input.
ORCID IDs
Marc De Meyer - https://orcid.org/0000-0003-0755-2898
Luis Bota - https://orcid.org/0000-0001-5392-0554
Massimiliano Virgilio - https://orcid.org/0000-0002-1323-6886
REFERENCES
Burgess N, D'Amico Hales J, Underwood E, Dinerstein E, Olson D, Illanga I, Schipper H, Ricketts T, Newman K. 2004. Terrestrial Ecoregions of Africa and Madagascar: A Conservation Assessment. Washington: Island Press. [ Links ]
Cogan BH, Munro HK. 1980. Family Tephritidae. In: Crosskey RW, editor. Catalogue of the Diptera of the Afrotropical Region. London: British Museum (Natural History). p. 518-554. [ Links ]
Copeland RS. 2007. On the occurrence of Bistrispinaria, grass-breeding fruit flies (Diptera, Tephritidae), in Kenya, with an addition to the tephritid checklist of Kakamega Forest. Journal of East African Natural History. 96: 95-102. https://doi.org/10.2982/0012-8317(2007)96[95:OTOOBG]2.0.CO;2 [ Links ]
Copeland RS, Okeka W, Freidberg A, Merz B, White IM, De Meyer M, Luke Q. 2005. Fruit flies (Diptera, Tephritidae) of Kakamega Forest, Kenya. Journal of East African Natural History. 94: 247-278. https://doi.org/10.2982/0012-8317(2005)94[247:FFDTOK]2.0.CO;2 [ Links ]
Correia ARI, Rego JM, Olmi M. 2008. A pest of significant economic Importance detected for the first time in Mozambique: Bactrocera invadens Drew, Tsuruta & White (Diptera: Tephritidae: Dacinae). Bolletino di Zoologia Agraria e di Bachicoltura Serie II. 40: 9-13. [ Links ]
Cugala D, Ekesi S, Ambasse D, Adamu RS, Mohamed SA. 2014. Assessment of ripening stages of Cavendish dwarf bananas as host or non-host to Bactrocera invadens. Journal of Applied Entomology. 138: 449-457. https://doi.org/10.1111/jen.12045 [ Links ]
Cugala DR, De Meyer M, Canhanga L. 2016. Chapter 24 Integrated management of fruit flies - Case studies from Mozambique. In: Ekesi S, Mohamed SA, De Meyer M, editors. Fruit Fly Research and Development in Africa - Towards a Sustainable Management Strategy to Improve Horticulture. Switzerland: Springer Verlag; p. 531-552. https://doi.org/10.1007/978-3-319-43226-7_24
De Meyer M. 2006. A systematic revision of the African fruit fly genus Carpophthoromyia Austen (Diptera, Tephritidae). Zootaxa. 1235: 1-48. https://doi.org/10.11646/zootaxa.1235.L1 [ Links ]
De Meyer M. 2009. Taxonomic revision of the genus Perilampsis Bezzi (Diptera: Tephritidae). Journal of Natural History. 43: 2425-2463. https://doi.org/10.1080/00222930903207868 [ Links ]
De Meyer M, Copeland RS. 2005. Description of new Ceratitis MacLeay (Diptera, Tephritidae) species from Africa. Journal of Natural History. 39: 1283-1297. https://doi.org/10.1080/00222930400004347 [ Links ]
De Meyer M, Freidberg A. 2005. Revision of the fruit fly genus Capparimyia (Diptera: Tephritidae). Zoologica Scripta. 34: 279-303. https://doi.org/10.1111/j.1463-6409.2005.00195.x [ Links ]
De Meyer M, Copeland RS, Lux S, Mansell M, Wharton R, White IM, Zenz N. 2002. Annotated check list of host plants for Afrotropical fruit flies (Diptera: Tephritidae) of the genus Ceratitis. Zoologische Documentatie Koninklijk Museum voor Midden Afrika. 27: 1-92. [ Links ]
De Meyer M, White IM, Goodger KFM. 2013. Notes on the frugivorous fruit fly (Diptera: Tephritidae) fauna of western Africa, with description of a new Dacus species. European Journal of Taxonomy. 50: 1-17. https://doi.org/10.5852/ejt.2013.50 [ Links ]
De Meyer M, Delatte H, Mwatawala M, Quilici S, Vayssières JF, Virgilio M. 2015. A review of the current knowledge on Zeugodacus cucurbitae (Coquillett) (Diptera, Tephritidae) in Africa, with a list of species included in Zeugodacus. ZooKeys. 540: 539-557. https://doi.org/10.3897/zookeys.540.9672 [ Links ]
De Meyer M, Majubwa RO, Biyusa KA, Vandenbosch M, Virgilio M, Mwatawala M. 2023. A first checklist of the dacine fruit flies (Diptera, Tephritidae, Dacinae) of Tanzania. Journal of East African Natural History. 112 (1): 1-19. https://doi.org/10.2982/028.112.0101 [ Links ]
Doorenweerd C, Leblanc L, Norrbom AL, San Jose M, Rubinoff D. 2018. A global checklist of the 932 fruit fly species in the tribe Dacini (Diptera, Tephritidae). ZooKeys. 730: 19-54. https://doi.org/10.3897/zookeys.730.21786 [ Links ]
Drew RAI, Yuval B. 1999. The evolution of fruit fly feeding behavior. In: Aluja M, Norrbom AL, editors. Fruit files (Tephritidae): Phylogeny and Evolution of Behavior. Boca Raton, USA: CRC Press; p. 731-749. https://doi.org/10.1201/9781420074468.ch27 [ Links ]
Drew RAI, Tsuruta K, White IM. 2005. A new species of pest fruit fly (Diptera: Tephritidae: Dacinae) from Sri Lanka and Africa. African Entomology. 13: 149-154. [ Links ]
EFSA. 2020. Pest categorization of non-EU Tephritidae. EFSA Journal. 18(1): 5931. [ Links ]
Ekesi S, De Meyer M, Mohamed SA, Virgilio M, Borgemeister C. 2016. Taxonomy, ecology, and management of native and exotic fruit fly species in Africa. Annual Review of Entomology. 61: 219-238. https://doi.org/10.1146/annurev-ento-010715-023603 [ Links ]
Garcia FRM, Bandeira RR. 2011. Biodiversidade de moscas-das-frutas (Diptera, Tephritidae) em Mozambique. Revista Eletronica Acolhendo a Alfabetização nos Paises de Lingua Portuguesa. 5: 24-44. [ Links ]
Hancock DL. 1999. Grass-breeding fruit flies and their allies of Africa and Asia (Diptera: Tephritidae: Ceratitidinae). Journal of Natural History. 33: 911-948. https://doi.org/10.1080/002229399300155 [ Links ]
Korir JF, Affognon D, Ritho CN, Kingori WS, Irungu P. 2015. Grower adoption of an integrated pest management package for management of mango-infesting fruit flies (Diptera: Tephritidae) in Embu, Kenya. International Journal of Tropical Insect Science. 35: 1-10. https://doi.org/10.1017/S1742758415000077 [ Links ]
Lux SA, Copeland RS, White IM, Manrakhan A, Billah MK. 2003. A new invasive fruit fly species from the Bactrocera dorsalis (Hendel) group detected in East Africa. Insect Science and its Application. 23: 355-360. [ Links ]
Manrakhan A, Venter JH, Hattingh V. 2015. The progressive invasion of Bactrocera dorsalis (Diptera: Tephritidae) in South Africa. Biological Invasions. 17: 2803-2809. https://doi.org/10.1007/s10530-015-0923-2 [ Links ]
Monteiro R. 1891. Delagoa Bay: Its Natives & Natural History. London, UK: George Philip & Son. https://doi.org/10.5962/bhl.title.17539 [ Links ]
Munro HK. 1984. A taxonomic treatise on the Dacidae (Tephritoidea, Diptera) of Africa. Entomology Memoir 61: 1-313. [ Links ]
Norrbom AL, Carroll LE, Thompson FC, White IM, Freidberg A. 1999. Systematic database of names. Myia. 9: 65-299. [ Links ]
Starkie ML, Cameron SL, Krosch MN, Phillips MJ, Royer JE, Schutze MK, Strutt F, Sweet AD, Zalucki MP, Clarke AR. 2022. A comprehensive phylogeny helps clarify the evolutionary history of host breadth and lure response in the Australian dacini fruit flies (Diptera: Tephritidae). Molecular Phylogenetics and Evolution. 172: 107481. https://doi.org/10.1016/j.ympev.2022.107481 [ Links ]
Vasse G. 1909. Trois années de chasse au Mozambique. Paris, France: Librairie Hachette. https://doi.org/10.2307/199685 [ Links ]
Virgilio M, De Meyer M, White IM, Backeljau T. 2009. African Dacus (Diptera: Tephritidae): Molecular data and host plant association do not corroborate morphology based classifications. Molecular Phylogenetics and Evolution. 51: 531-539. https://doi.org/10.1016/j.ympev.2009.01.003 [ Links ]
Virgilio M, White IM, De Meyer M. 2014. A set of multi-entry identification keys to African frugivorous flies (Diptera, Tephritidae). ZooKeys. 428: 97-108. https://doi.org/10.3897/zookeys.428.7366 [ Links ]
von Röder V. 1885. Ueber die Dipteren-Gattung Ceratitis Mac Leay. Berliner Entomologische Zeitschrift. 29: 132-137. https://doi.org/10.1002/mmnd.18850290119 [ Links ]
White IM. 2006. Taxonomy of the Dacina (Diptera: Tephritidae) of Africa and the Middle East. African Entomology Memoir. 2: 1-156. [ Links ]
White IM, Copeland RS, Hancock DL. 2003. Revision of the afrotropical genus Trirhithrum Bezzi (Diptera: Tephritidae). Cimbebasia. 18: 71-137. [ Links ]
White IM, Elson-Harris MM. 1994. Fruit Flies of Economic Significance: their Identification and Bionomics. Wallingford, UK: CAB International. [ Links ]
White IM, Goodger KFM. 2009. African Dacus (Diptera: Tephritdae); new species and data, with particular reference to the Tel Aviv University collection. Zootaxa. 2127: 1-49. https://doi.org/10.11646/zootaxa.2127.1.1 [ Links ]
 Correspondence:
Correspondence:
Marc De Meyer
Email: demeyer@africamuseum.be
Received: 07 February 2023
Accepted: 02 May 2023