Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 n.6 Johannesburg Jun. 2012
JOURNAL PAPER
Sulphating roasting of copper-cobalt concentrates
J. Güntner; J. Hammerschmidt
Outotec, Germany
SYNOPSIS
Most copper/cobalt ores from the Central African Copperbelt contain sulphidic compounds, which are not extractable in direct leaching. Roasting provides the possibility to transfer the valuable components into leachable compounds. In an operation window defined by temperature and off-gas composition it is possible to selectively oxidize iron sulphides to haematite and copper/cobalt sulphides to sulphates.
Selective sulphating of the valuable minerals is an important condition for the subsequent leaching stage. The dissolved copper and cobalt are recovered by solvent extraction and electrowinning. This technology has been part of an established process for many decades.
The concentrates from recent projects on the Copperbelt have lower sulphur contents, and frequently higher copper contents, than those from older projects. The paper shows the consequences of this trend for plant operation and explains useful counter actions by means of case studies. Autothermal combustion is ensured even at very low sulphur contents.
Off-gas treatment has so far been based on SO2 removal through sulphuric acid production. The generated acid is used to make up acid losses in the hydrometallurgical plant section. This well-proven process combination is still the preferred route if the feed sulphur content is high enough; however, some cases may require different off-gas cleaning concepts without compromising on clean air quality.
Roasting in a fluid-bed furnace remains the core of the technology. Fluid-bed roasters are easy to operate and provide an excellent control of the calcine quality. Different feeding and heat-recovery systems are used to provide a tailor-made solution for each project.
Outotec built it first sulphatizing roaster in Zambia about 30 years ago. At that time, the roasting of metal sulphides was already one of the main technologies in the company. Outotec has developed roasting solutions for copper, gold, zinc, lead, molybdenum, and pyrite ores. The continuously expanded and upgraded research facilities provide an excellent basis for developing sustainable, energy-efficient, and economic solutions for copper and cobalt production in Africa.
Keywords: sulphating roasting, copper concentrate, copper-cobalt concentrate, copper sulphate, copper-cobalt sulphate, chalcosite, chalcopyrite, Copperbelt.
Introduction
Sulphating roasting is a unit operation in the production process for copper and cobalt from sulphidic ores. The simplified block diagram in Figure 1 shows that roasting is the first stage in the process flow sheet. The objective of roasting is a selective sulphation of copper and cobalt, while ferrous minerals and components are oxidized to haematite. The sulphates are dissolved in the leaching stage. Calcine leaching is often carried out with spent acid to improve leaching kinetics and to recover also the small portion of copper oxides that are unavoidably generated during roasting and that would otherwise be lost.

Under these conditions iron remains practically insoluble and can be separated with the tailings from the copper- and cobalt-containing liquor. Extraction of copper and cobalt is carried out by solvent extraction with subsequent electrowinning (SX-EW). The roasting off-gas is used for sulphuric acid production. This production process has been in operation for several decades. It is particularly suitable for cobalt-containing copper ores. Process control is uncomplicated and operation is profitable at low capacities.
Reactions
The sulphation reaction occurs in three main steps. In the first step the metal sulphides are oxidized to metal oxides. Me denotes mainly Cu, Co, and Fe but also Ni, Ca, and Mg.

In the second step, sulphur dioxide is oxidized to sulphur trioxide. This reaction is supported by copper and iron acting as catalysts.

Finally, the metal oxides react with sulphur trioxide to form sulphates and basic sulphates.

The direct sulphation of metal sulphides is also possible. Oxygen excess is important for all sulphation reactions.

Figure 2 shows the predominance phase diagram for the Cu-O-S system. The off-gas contains typically 5-8 vol.% of oxygen in order to shift the reaction to the formation of copper sulphate. High SO2 contents are also favourable for the reaction; however, the SO2 level depends on the sulphur content of the feed material. It is possible to intensify the sulphation reaction by addition of NaSO4 as it was practised in the past2.
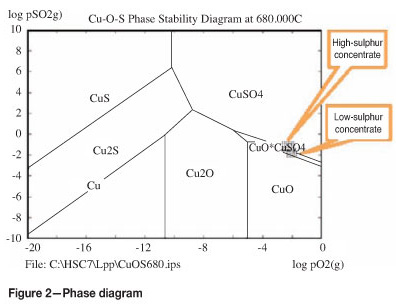
The typical temperature range is 650-700°C. Testwork in Outotec's R&D centre confirmed that above 650°C, Fe2O3 is the predominant stable iron compound. Pre-existing iron sulphates decompose to haematite, while a substantial decomposition of copper sulphates starts only at 700°C. At 680°C, 93 per cent of the copper exists as sulphate, while at 720°C this proportion drops below 50 per cent. Cobalt is less sensitive. Even at 720°C more than 90 per cent remains in the sulphate form. More than 95 per cent of the iron sulphates are decomposed at 680°C. A reliable temperature control and as small a spread of temperature as possible in the furnace are essential for high copper recovery.
Roasting process
Roasting is carried out in a bubbling fluid bed. Figure 3 shows the elements of the roasting area. The furnace consists of a cylindrical bottom section that contains the fluidized bed, a conical transition section, and an expanded freeboard. The feed material is injected as slurry by means of compressed air through slurry lances into the fluidized bed. In order to avoid material build-up at the furnace walls as well as abrasion of the furnace refractory lining, the injected slurry should not impinge on the furnace walls. Lance position, direction, and injection pressure are designed and adjusted accordingly. The slurry is atomized when it contacts the fluidized bed to ensure homogeneous material distribution over the full furnace area.

The roasting process is autothermal. The sulphides in the feed serve as fuel and as reagent to form sulphates. The roasting temperature is kept within a narrow range of ± 10°C.
As explained in the previous section, the temperature window is 650-700°C. The slurry density is adjusted to slightly higher than that required for autothermal operation. Fine tuning of the temperature is achieved by water or liquor addition into the slurry at the inlet to the slurry lances.
Roasting air enters the furnace through the nozzle grate. The height of the fluidized bed is 1 to 1.5 m, corresponding to a windbox pressure of 180-230 mbar. The calcine is discharged via an overflow weir. Entrained dust particles leave the furnace with the off-gas and are separated in a cyclone stage. The small dust particles contain more sulphate-compounds than the furnace bed material, due to the different process conditions in the freeboard. This fact applies for copper, cobalt, and unfortunately also for iron. In order to limit the content of iron sulphates, the dust is therefore separated in the cyclone stage and returned to the furnace. Owing to its high sulphate content, the dust is sticky and its flow behaviour is problematic.
A boiler is standard in some other roasting applications to recover heat from the off-gas. However, it is not used in sulphating roasting because of the low off-gas temperature and the stickiness of the dust. The off-gas temperature is reduced with water in one or two quench stages. Slurry feeding and water quenching of the off-gas generates rather high volumes of off-gas. The contained water vapour has to be removed by condensation in a gas cooling stage. A sulphuric acid plant is standard for the final off-gas treatment.
The majority of the calcine is removed from the roaster via a height-adjustable bed overflow and is discharged to the calcine quench tank. In other roasting applications the calcine is cooled by indirect heat transfer in a water-cooled fluid-bed cooler. Indirect calcine cooling is usually applied if the calcine is to be conveyed and stored dry or if water is short. In endothermic processes, calcine cooling is a source of heat recovery to reduce the fuel consumption. Gold ore roasters that treat the whole ore instead of a concentrate are endothermic owing to the low sulphur contents of 2-5 per cent.
Conventional sulphatizing roasting
Feedstock examples are shown in Table I. Concentrate A is typical for most realized projects so far. The copper content is usually less than the sulphur content. The iron content varies between 15 and 25 per cent. Cobalt is always present in the low percentage range. Concentrates of type B were rather atypical in the past; however, they have been appearing in some recent projects. The copper contents are much higher and the sulphur contents much lower compared with concentrate A. Copper is between 30 and 40 per cent and sulphur is well below 20 per cent. Iron contents can be as low as 5 per cent. Concentrate A contains mainly chalcopyrite with a low portion of chalcocite whereas in concentrate B chalcocite predominates over chalopyrite. This variation affects the heat balance, the calcine composition, and the off-gas treatment.

The data in Table II represents the roasting operation with type A concentrate. Copper sulphide is converted by more than 90 per cent to CuSO4 and CuO.CuSO4. The ratio between the sulphate and the basic sulphate is usually around 2:1. The calcine contains to a small extent CuO and traces of CuS. More than 95 per cent of the iron is in the oxide form. The rest appears mainly as sulphate. The iron sulphates are soluble in the leaching stage. Up to 5 per cent of the iron is usually dissolved; the rest remains in solid form. More than 95 per cent of the cobalt is sulphatized; the rest is oxide. The selected example is based on a feed rate of 15 t/h (dry basis). Slurry feeding at 65-70 wt% solids is usual.

The required roasting air flow is 32 000 Nm3/h. The off-gas flow rate after the roaster is 39 000 Nm3/h. It increases to more than 40 000 Nm3/h in the off-gas quench stages and is reduced to 30 000 Nm3/h after wet gas cooling. The SO2 content of the off-gas is 5 per cent,, which makes it suitable for sulphuric acid production. The example reflects the base case for sulphating roasting. Copper and cobalt recoveries are usually above 90 per cent.
Adaptation to different feed materials
The sulphur distribution between calcine and off-gas is determined mainly by the temperature, which in turn should be between 650 and 700 °C to promote formation of iron oxides and of copper/cobalt sulphates. The ratio of Cu+Co/S is typically 1.2 to 1.3 for concentrate A types. This ratio is determined by the mineralogy of the ore. High chalcocite content causes lower Cu/S ratios. Concentrates of type B have mostly Cu+Co/S-ratios below 1. The consequence is that the calcine contains a higher portion of copper and cobalt oxides and that the SO2 content in the off-gas drops well below the limit for autothermal sulphuric acid production.
The phase diagram in Figure 2 shows that the operating point is shifted towards the stability fields of basic sulphates and, even, of copper oxides. The different calcine composition affects the conditions and the sulphur balance in the leaching and SX-EW process stages.
Heat-balance calculations show that autothermal roasting is not sustainable at a slurry solid content of 65 per cent. The following possibilities exist to ensure autothermal roasting at low sulphur contents:
Higher slurry solid content
Solid feeding instead of slurry feeding
Preheating of roasting air
Use of oxygen-enriched roasting air.
A higher slurry solid content is a possibility if the sulphur deficit for autothermal roasting is only small. The physical limits of thickening and slurry pumping have to be considered. Solid contents above 70 per cent are sometimes difficult to achieve in conventional thickening, and slurry transport becomes critical at high solid contents. Moreover it is observed that atomization of the slurry at such high solid contents is difficult.
Solid feeding requires filtration of the slurry. The feeding system has to be changed. Figure 4 shows feeding with slinger belts, which have been common practice in Outotec zinc and pyrite roasters for a long time. The material is fed directly onto the fluid bed. Dust entrainment is therefore limited. The feed material can be moistened with spray water on the feeding belt conveyor. This helps to control the temperature in the furnace. Considerable experience has been gained in zinc ore and pyrite roasting. The material enters the furnace through feed ports and is homogeneously distributed in the furnace. The furnace has to be under slight negative pressure to avoid escape of roasting gases through the feed ports. In the event that plant operation is disturbed, automatic gates shut the feed ports and feeding is stopped.

Slinger belt feeding is used even for very fine concentrates with 80 per cent below 45 μm. If required, a microgranulation stage can be incorporated in the feeding system area in order to tackle the problem of superfines.
Preheating of the roasting air is common for endothermic processes such as calcination of aluminium hydrate. In order to ensure autothermal roasting, the heat contained in the calcine is transferred in calcine cooling to the roasting air. Figure 5 shows a typical fluid-bed cooler solution as used in Outotec's fluid-bed processes for several decades. To date such installations are uncommon in roasting applications as roasting is always exothermic. For endothermic processes such as aluminum hydrate calcination or decomposition of sulphates they are common. The example in Figure 5 is from a gold roasting plant in the USA. As whole-ore roasting was applied the process was endothermic. The sulphur contents whole-ore roasting are typically below 5 per cent, which is not sufficient for autothermal roasting. Figure 6 shows a further example of a fluid-bed cooler with heat recovery for a roasting application. The fluidizing air is de-dusted in a cyclone and the dust is directed together with the cooler discharge to the calcine quench tank.


Preheating up to 400°C is common. Process calculations for roasting of concentrate B show that the roasting air needs to be preheated to 330°C to ensure autothermal roasting with slurry feeding at 65-70 wt% solids. The calcine is cooled to 250°C. Further calcine cooling is possible; however, owing to the weak heat transfer at low temperatures it is more economical to quench the calcine once its temperature drops below 200°C and to use the contained heat in calcine leaching. The fluid-bed cooler contains air bundles through which the roasting air is directed countercurrent to the calcine flow. The cooler is stationary; it contains no mechanical parts, which keeps maintenance at a minimum. A fan is required with a capacity around 2000 Nm3/h for fluidizing the calcine. This air stream is heated in the cooler and is introduced into the furnace as secondary air at the conical furnace section. Table III shows that the roasting air requirement is substantially lower than for case A. The off-gas volumes are also less. The SO2 content is 1-2 vol.%, however, which is not sufficient for sulphuric acid production. The SO2 content should not be less than 4 vol. % for economic acid production. At SO2 contents below 1 vol.% simple scrubber solutions can be applied. And in the SO2-range of 2-4 vol. %, special solutions are required that are costly either in terms of energy or chemicals.
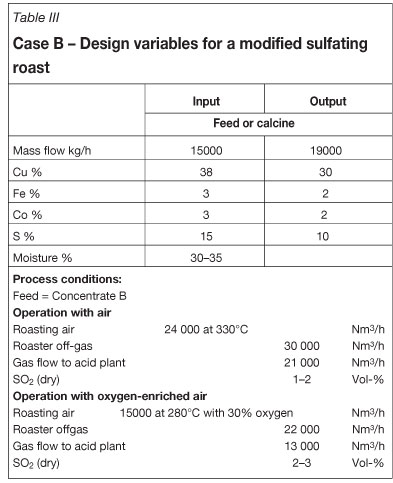
We have investigated oxygen enrichment as it is used in a number of roasting plants. The data is shown in Table III. At an oxygen enrichment of 30 per cent the air needs to be preheated to 280°C for autothermal roasting. No benefit is derived as the expense for the calcine cooler remains unchanged. The SO2 content increases to 2-3 vol.%, which is still insufficient for sulphuric acid production or, more precisely, for acid production without additional sulphur combustion. The off-gas volume decreases considerably to only 13 000 Nm3/h.
Taking all of the aforementioned possibilities into consideration, we find that preheating of the roasting air seems to be the most profitable solution for sulphating roasting of concentrates with high copper and low sulphur contents. The required equipment is well known and is in operation in numerous Outotec fluid-bed plants. Roaster operation is practically unchanged with slurry feeding at common solid contents. The off-gas volume is low; however, it requires a scrubber solution for SO2 removal. The high water content at slurry feeding is supposed to promote the formation of copper sulphates. Feeding of a solid filter cake at 8 per cent moisture and temperature control by water addition would provide a process off-gas with lower water content; however, the off-gas composition is anyway quite similar to an operation with slurry feeding. In numerous pilot tests we could not discern a clear trend, whether slurry feeding supports the formation of metal sulphates compared to feeding of filter cake.
Test work and plant design
For exact plant design, roasting tests are necessary. Outotec operates a number of fluid-bed test units in their R&D centre in Frankfurt. Pilot tests in continuous units permit detailed plant design provision of performance guarantees. Figure 7 shows the pilot plant that was used in designing a number of Outotec roasters. All Outotec gold roasters (Cortez and Carlin in the USA, Kalgoorlie in Australia, Minahasa in Indonesia, and Syama in Mali), as well as a number of copper and pyrite roasters, have been designed following tests conducted in this plant. The pilot plant allows one to extract information about product quality and off-gas composition.
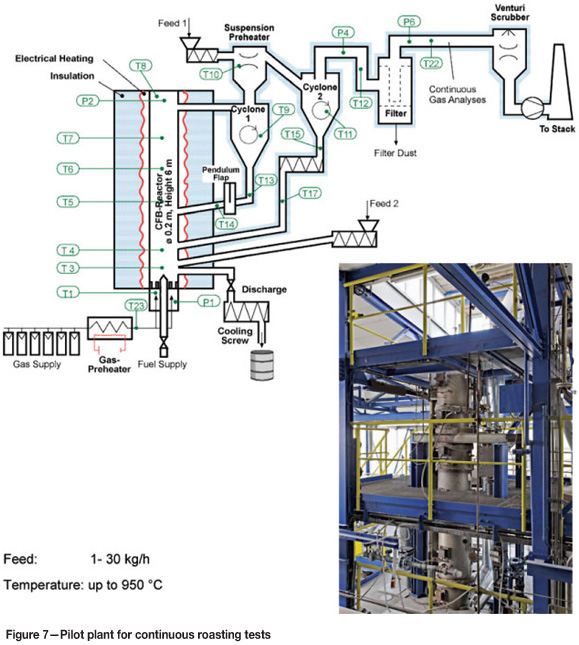
Leaching tests can either be carried out at our R&D centre or by the project owner. For the more common zinc and pyrite roasting applications, testwork is mostly not required as our database built up over the years permits us to design a plant without tests. Other applications, such as gold and copper roasting, are different. The number of built plants is fewer and the variation in feedstock materials is wider.
Outotec has built roasting furnaces with nozzle great areas from 15 to 138 m2. The number of plants for sulphating roasting is low. Most have a furnace area of about 50 m2. Outotec has built roasters below 50 m2, mostly for specific duties; however, these are no longer in operation.
Outotec build several modularized roasting furnaces in the early years of their development. For specific projects it might be better choosing a small furnace. It is important therefore to keep the ability not only to design and build bigger plants, but also to develop tailor-made solutions for economic operation on a small scale.
References
1. Pawlek, F. Metallhüttenkunde. Walter de Gruyter, Berlin, New York, 1983. [ Links ]
2. Palperi, M. and Aaltonen, O. Sulphatizing roasting and leaching of cobalt ores at Outokumpu Oy. Journal of Metals, vol. 34, 1971. pp. 34-38. [ Links ]













