Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.124 n.12 Johannesburg Dec. 2024
https://doi.org/10.17159/2411-9717/702/2024
HYDROMETALLURGY PAPERS
Optimization of chromium precipitation from ferrochrome leach solutions
T.V. Nyangadzayi; F. Ntuli
Botswana International University of Science and Technology, Botswana. ORCiD: T.V. Nyangadzayi: http://orcid.org/0009-0005-9698-9611; F. Ntuli: http://orcid.org/0000-0003-4045-902X
ABSTRACT
Chromium green is an essential metal with various applications in the paints, construction, electronics, and aerospace industries. The main source of chromium green is the carbo-thermic reduction of chromite ores, however, chromite ores are being heavily depleted on a global scale, hence the need to find alternative or secondary sources for the chromium. In order to meet the global demand for chromium there is a need to implement principles of the sustainable circular economy to keep chromium within the material loop. Ferrochrome slag and stainless steel slags are secondary resources that are potential sources of chromium. In this work, recovery of chromium from ferrochrome slag was investigated. The ferrochrome slag was alkaline leached using sodium hydroxide- sodium hypochlorite solution to reduce the use of potent reagents that have a potential negative environmental impact.
The leachate produced by oxidative alkaline leaching of ferrochrome slag contained hexavalent chromium, which was used to recover the extracted chromium through a two-stage process involving reduction and precipitation. Reduction of hexavalent Cr to trivalent Cr was investigated using anhydrous FeSO4 at concentrations of 5 g/L, 10 g/L, and 15 g/L. Precipitation was conducted by dosing the reduced leach solution with 1 M sodium hydroxide at dosages of 5 ml/L, 10 ml/L, and 15 ml/L. The experiments were designed using the Taguchi (L9) orthogonal array and analysed according to the ANOVA, general linear model. The optimized conditions for the reduction step were ferrous sulfate dosage of 5 g/L with a pH of 12, and for the precipitation step, 1 M sodium hydroxide at a dosage of 15 ml/L with a setting time of 90 minutes and pH of 7. Physical changes were observed during the two-step chromium recovery, with solution changing from a yellowish colour to dark green colour indicating completion of the reaction. The chromium recovery was found to be 93.3%.
Keywords: ferrochrome slag, precipitation, reduction, chromium recovery, alkaline leaching
Introduction
Chromium green is an essential element with various applications in the paints, food, and tanneries industries (Table I). Its market share in 2022 was 50.7% or USD631 million, which was projected to increase by 5.2% to USD864.9 million in 2028, as the chromium green market demand rises (Horckmans et al., 2019; Pacific, America and Forecasts, 2020; Pfaff, 2022). The primary source of chromium is the carbothermic reduction of the chromite ores (Holappa and Xiao, 2004; Sahu, Biswas and Kapure, 2016). The ferrochrome slag and stainless steel slags are secondary sources, with a potential to be further processed to recover chromium. This is of benefit to the environment as waste valorization of the slags will eliminate the release of hexavalent chromium, which is carcinogenic, mutagenic, and toxic. Hexavalent chromium has been recovered as chromium hydroxide, which is then calcined to chromium green. The ferrochrome slag residue after alkaline leaching can then be utilized in the road and construction industry (Erdem et al., 2005; Hariharan and Murali Krishna, 2013; Acharya and Patro, 2016).
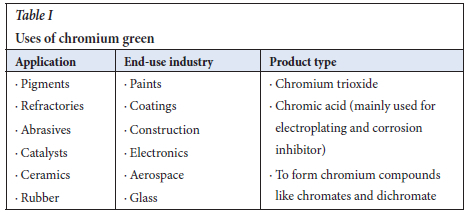
The ferrochrome slag was alkaline leached and the main challenges are the presence of iron and aluminium, which co-dissolute and co-precipitate with the chromium (Kocaoba and Akcin, 2004; Kim et al., 2016). There are many solution concentration, purification, and metal recovery methods that may be implemented, however, not all methods are applicable for liquors produced from the oxidative alkaline leaching process. The commonly used methods are solvent extraction, ion exchange, and precipitation (Kanari et al., 2020). Studies to recover chromium(III) and chromium(VI) have been done mainly for recovering chromium from waste waters from industries like tanneries (Mane et al., 2016, Kocaoba and Akcin, 2004). Precipitation as a method of recovery does produce high recoveries of chromium due to its high selectivity in creating a pure and homogeneous product whilst retaining iron and aluminium in the ferrochrome leach solution (Gheju, 2018).
Precipitation is selected as the metal recovery step, as it focuses mainly on recovering the aqueous chromium into a purified solid phase of chromium, with the exception that in some cases, solution purification will be required since precipitation is a nonselective technique, thus other components may co-precipitate from the solution. Alkaline leaching of ferrochrome slag favours the formation of Cr(VI), which is toxic and mutagenic and a major threat to the environment due to its mobility. Many precipitating agents may be considered for Cr(VI) ions, namely: barium chloride, lead sulfate, sodium hydroxide, calcium hydroxide, calcium carbonate, sodium carbonate, sodium hydrosulfide, iron sulfide, and sodium borohydride. However, some compounds like barium chloride precipitate chromium in its hexavalent form, which is a major problem (Ramakrishnaiah and Prathima, 2012). Kim et al., (2016), investigated the precipitation of chromium by barium chloride to form BaCrO4 after oxidative alkaline leaching of stainless steel slag to recover chromium. The method achieved recoveries of above 90% with the following Equation [1].
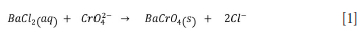
To recover chromium in its most stable form, which is environmentally friendly, a conversion step from Cr(VI) to Cr(III) should be undertaken and followed by precipitation to hydroxyl species Cr (OH)3. This method has high recoveries of chromium and is an environmentally friendly method. The conversion step is known as the reduction step where various reducing agents may be used (Fang et al., 2012). These reducing agents are hydrogen sulfide, hydrogen peroxide, sulphur dioxide, sodium metabisulphite, and ferrous sulfate (Ramakrishnaiah and Prathima, 2012). The commonly used reducing agents are sulphur dioxide and sodium metabisulphite at pH = 2 with a retention time of 30-40 min. Ferrous sulfate, however, is used in alkaline/neutral conditions. H2S and SO2 are very toxic reagents and come with environmental problems (Shen and Forssberg, 2003). Ferrous(II) sulfate is abundant, cost-effective, and has no complicated chemistry in the reduction of Cr(VI) and the reaction is presented in Equation [2] (Lokothwayo, 2007):
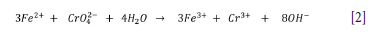
After the reduction step, precipitation is the last step in chromium recovery. There are various hydroxide precipitating agents, mainly sodium hydroxide and calcium hydroxide (Baijnath et al., 2014). The factors that affect precipitating reaction kinetics that were considered in this study are pH, chromium(VI) concentration, precipitant dosage, and settling time. For high recoveries of chromium(III), pH values during the chemical precipitation step should be in the neutral value of pH = 7 (Fang et al., 2012). Chemical precipitation cannot be conducted at low concentrations of Cr(III) as it is not economic due to the high costs of chemicals and reagents used for precipitations and also the removal of the precipitates after the process (Falagán, Grail and Johnson, 2017). The Cr(III) produced during alkaline leaching is concentrated and therefore will have high recoveries of chromium(III) hydroxide (Ramakrishnaiah and Prathima, 2012).
The precipitant dosage affects the recovery efficiency of Cr(III), and an optimum dosage should be used. For NaOH low dosages are required for high recoveries compared to Ca(OH)2. NaOH is more soluble than Ca(OH)2, which is a weak base, and reacts rapidly with Cr(III) (Baijnath et al., 2014). The precipitate yield increases with an increase in settling time, as this allows for coagulation of small precipitate particles (Baijnath et al., 2014). The major advantage of this precipitation method is that Cr(OH)3 has limited mobility and is safe for the environment as it is an essential nutrient in the human diet (Beukes, Pienaar and Lachmann, 2000). In selecting the precipitating agent, the costs and effectiveness of the precipitating agent were considered. NaOH is readily available and cost-effective at USD0.30/kg of NaOH, with high recoveries of above 99% and solubility in water of 111g/100ml at 25°C (Ramakrishnaiah and Prathima., 2011), whereas Ca(OH)2 is readily available at USD0.45/ kg and operates at low pH values of 2 with solubility in water at 0.173g/100ml at 25°C (Baijnath et al., 2014). Sodium hydroxide was selected as the precipitating agent. The overall equation (Equation [3]) for chemical precipitation using NaOH as the precipitant is:
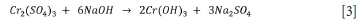
The aim of the study was to optimize the reduction and precipitation of Cr from ferrochrome leach solutions using ferrous sulfate as a reducing agent and sodium hydroxide as a precipitating agent.
Materials and methods
Materials
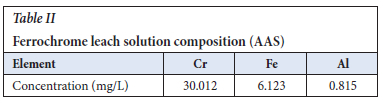
5.0 g Fe-Cr slag was weighed and premixed with 20 ml of NaOH for homogeneity and 20 ml of NaOCl. The slurry formed was reacted isothermally in an alumina crucible in a temperature-controlled heating oven without stirring for 6 hours. After cooling the slurry was water-leached with distilled water for 1 hour at 25°C. The pregnant liquor was collected by filtration and analysed for Cr, Al, and Fe to check for Cr leachability through the use of a Thermo Fisher inductively coupled plasma atomic emission spectroscopy (ICP- AES), of which the results are shown in Table II
Ferrous sulfate was used for the reduction step in reducing Cr6+ to Cr3+ oxidation state. The sodium hydroxide at 1M was used for the precipitation step to precipitate the Cr3+ to chromium hydroxide. Chromium hydroxide was to be calcined to produce chromium green.
Experimental method
Ferrous sulfate was added to the pregnant liquor to reduce Cr(VI) to Cr(III). Ferrous sulfate was added at different concentrations (5 g/l, 10 g/l, and 15 g/l) to ensure complete reduction of the Cr(VI). The Cr(VI) was determined using diphenyl-carbazide as a reagent at a pH of 1 and analysed by a spectrophotometer at a maximum wavelength of 540 nm (Wiryawan et al., 2018). The reduced Cr was analysed by ICP-AES to determine the Cr recovered. The Cr(III) in the solution was then precipitated by NaOH at 1 M. The precipitate was left to settle at the bottom and separated from the solution. The spent solution was analysed by ICP-AES for the remaining Cr in the solution in order to determine Cr recovery (Gheju, 2018; Weng et al., 1994).
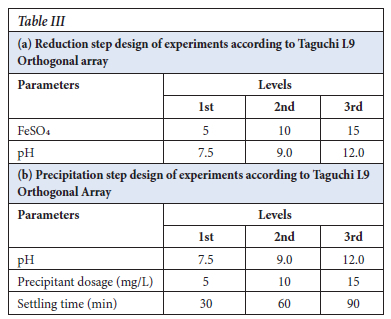
The reduction step (9 experiments) and precipitation step (9 experiments) for Cr recovery experiments were designed according to the Taguchi L9 orthogonal array with the effect of the following factors: reductant dosage, Cr6+ content in solution, precipitant dosage, pH, and settling time were investigated. Table III illistrates the Taguchi L9 orthogonal array design of experiments. The terms neutral mean pH range of 7-8, alkaline 8-10, and very alkaline 10-14.
Results and discussion
The recovery was divided into two stages namely reduction (hexavalent Cr to trivalent Cr) using anhydrous FeSO4 at different dosages, and precipitation using NaOH as the precipitant. The chromium, iron, and aluminium content (Table IVa and Table IV2b) was analysed using atomic absorption spectroscopy, AAS, and Thermo Scientific ICE 3000. The initial amount of chromium in the ferrochrome leach solution was found to be 30 mg/L. The Cr6+ was determined colourimetrically using diphenylcarbazide in an acid solution with a red to violet colour change as a positive test.
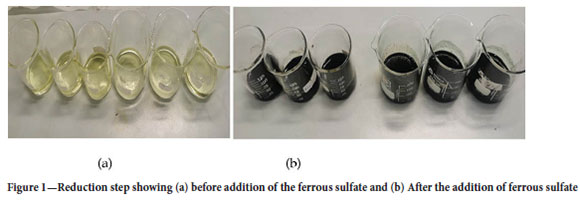
Reduction stage: The chromium ions in solution were found to be Cr6+, which are favourable in alkaline conditions. The Cr6+ ions were reduced to Cr3+ ions in the presence of anhydrous ferrous(ll) sulfate with the colour changing from yellow-greenish to dark green (positive colour change for Cr3+ ions), as shown in Figure 1.
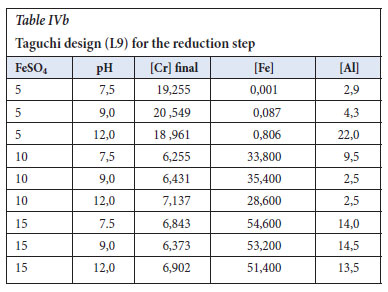
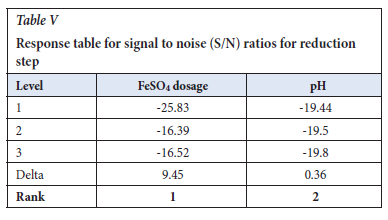
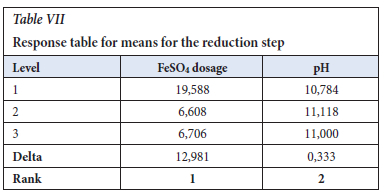
The Taguchi design was analysed using the Minitab 19 software, and the S/N ratios were calculated with the Delta being the difference between the highest response value from the lowest response values. The important factor was the ferrous sulfate dosage. The pH was in the range of 7.5-12.0 and it showed less significance as shown in Table V.
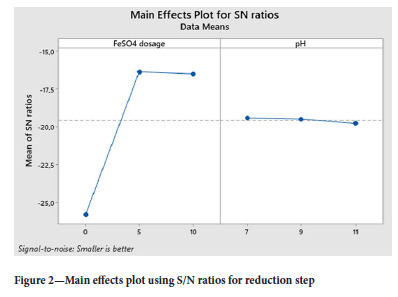
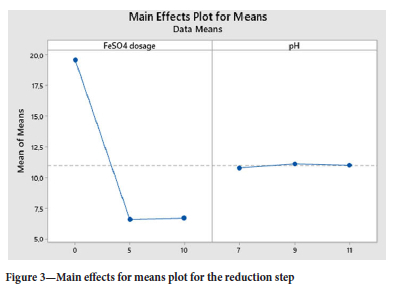
According to the main effects plot in Figure 2, the optimum conditions to effectively reduce the Cr6+ to Cr3+ were determined. The colour change was seen from yellow-greenish to dark green indicating completion of the reaction. The Cr6+ ions were analysed and the smaller the better approach was selected. The ferrous sulfate dosage of 5 g/L was observed as the optimum conditions at a pH of 7.5.
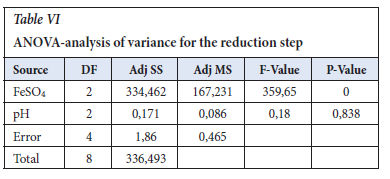
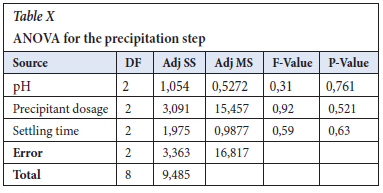
The ANOVA (in Table VI) identifies the significance and importance of each factor and ferrous sulfate p-value was 0.000, in which the Null hypothesis of p=0.05 confidence proves that the parameter is very significant in the reduction step reaction (Gülcan, Karahan and Gürmen, 2020). The response table for means was used to determine the importance of the individual parameter and it is in agreement with the S/N ratios values, which is that ferrous sulfate is the important factor with a Delta of 12.981 versus that of pH of 0.333 in Table VII. In Figure 3, the main effects plot does show that the pH is almost constant in the range of 7.5 to 12.0.
Precipitation step
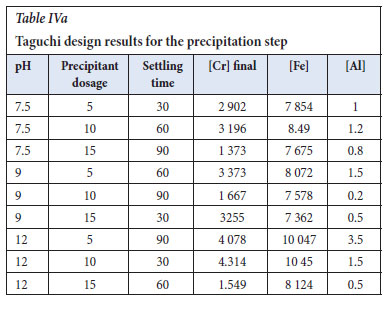
The experiments were designed according to Taguchi and analysed using ANOVA, and the Level 9 (33) orthogonal array was selected. The response table for signal to noise ratios (Table VIII), employing the optimization approach of smaller is better, shows the largest Delta as 5.073, thereby indicating precipitant dosage as the main important effect. The precipitant used was 1 M sodium hydroxide at room temperature. The settling time was the second factor, which has an effect on the precipitation step as small colloids were formed and required a lot of time to settle
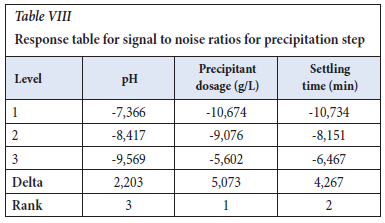
The response table for means (Table IX, agrees with the Table VIII for S/N ratios, with sodium hydroxide dosage as the main factor that affects the precipitation step.
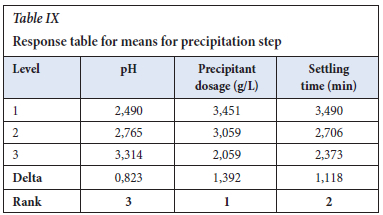
Figure 4 shows the main effects plot for the precipitation step, and the optimum conditions to effectively recover the chromium as chromium hydroxide are NaOH dosage of 15 ml/L at 1 M, the pH of 7, and settling time of 90 mins. As the reaction progresses, the increase in NaOH dosage results in Cr being reduced and precipitated into chromium hydroxide. The longer the settling period, the more the chromium is removed from the solution.
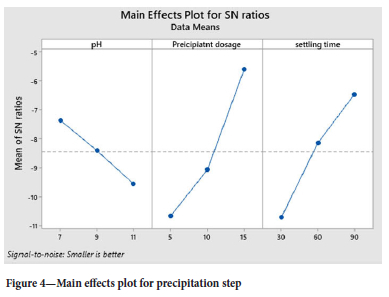
The Taguchi design was analysed according to the ANOVA, general linear model in Table X, and the p-values were very significant for NaOH dosage, pH, and settling time at 0.521, 0.761, and 0.630, respectively. The lower the value of p-value (near to zero) of the parameters, the more successful the precipitation step (Essa et al., 2022).
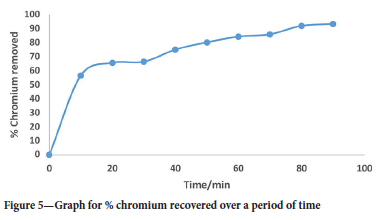
Chromium in solution was reduced, precipitated, and investigated according to Taguchi L9 (33) orthogonal arrays. The chromium concentration was measured at different intervals of 10, 20, 30, 40, 50, 60, 70, 80, and 90 minutes, using the AAS. The chromium recovery was found to be 93.3% from the solution. In the first 10 minutes, there was a rapid increase in chromium removed in solution, which is due to the fact that the reaction is very fast, and anhydrous ferrous sulfate and sodium hydroxide at 1 M were used.
These conditions were strongly alkaline and thereby improving the chromium removed from the solution. Figure 5 shows the recovery graph of chromium from the leachate solution. As the reaction progresses, the percentage recoveries of chromium started to stabilize and become almost constant at 93.3%, which was the highest recorded.
Conclusion
The hydroxide precipitation of chromium by the alkali-precipitating agent sodium hydroxide has proven to be effective. With chromium being amphoteric (soluble at both acidic and alkaline conditions) at a pH of 6-9, precipitation is favourable. The Fe3+ and Al3+ ions however, are effectively precipitated at a pH of 2 and 3, respectively. Due to Cr3+ ions being precipitated at a pH range of 6-9, the sodium hydroxide effectively precipitated chromium with minimal interference to co-precipitation from Fe3+ and Al3+ ions.
The recovery of chromium from the leachate was designed and analysed using Taguchi (L9 orthogonal array) and ANOVA respectively. The recovery of chromium from the leachate was calculated and found to be 93.3% at optimum conditions for the reduction step of ferrous sulfate dosage - 5 g/L and pH-7.5, and for the precipitation step: sodium hydroxide (1 M) dosage - 15 ml/L, pH - 7, and settling time - 90 minutes. These optimum conditions allowed for recoveries of the chromium from the solution at 93.3%. The chromium extracted was roughly 4.55 mg/g of slag.
References
Acharya, P.K., Patro, S.K. 2016. Utilization of ferrochrome wastes such as ferrochrome ash and ferrochrome slag in concrete manufacturing, Waste Management and Research, vol. 34, no. 8, pp. 764-774. doi: 10.1177/0734242X16654751 [ Links ]
Baijnath, L.L., Gautam, V., Yadav, V.L. 2014. A Comparative Study of the Removal Efficiency of Calcium Hydroxide and Sodium Hydroxide as Precipitating Agents for Chromium (III). Journal of Civil Engineering and Environmental Technology, vol. 1, no. 1, pp. 17-20. https://krishisanskriti.org/volimage/03Tul20151207216.pdf [ Links ]
Beukes, J.P., Pienaar, J.J., Lachmann, G. 2000. The reduction of hexavalent chromium by sulphite in wastewater - An explanation of the observed reactivity pattern. Water SA, vol. 26, no. 3, pp. 393-395. [ Links ]
Erdem, M. 2005. Hexavalent chromium removal by ferrochromium slag. Journal of Hazardous Materials, vol. 126, no. 1-3, pp. 176-182. doi: 10.1016/i.ihazmat.2005.06.017 [ Links ]
Essa, W. K. 2022. Polyethylene Terephthalate Nanofiber-Multi-Walled Carbon Nanotube Composite, vol. 25. doi: 10.3390/w14081242 [ Links ]
Falagán, C., Grail, B.M., Johnson, D.B. 2017. New approaches for extracting and recovering metals from mine tailings. Minerals Engineering, vol. 106, pp. 71-78. doi: 10.1016/i.mineng.2016.10.008 [ Links ]
Fang, H.X., Li, H.Y., Xie, B. 2012. 'Effective chromium extraction from chromium-containing vanadium slag by sodium roasting and water leaching. ISIJ International, vol. 52, no. 11, pp. 1958-1965. doi: 10.2355/isiiinternational.52.1958 [ Links ]
Gheju, M. 2018. Progress in understanding the mechanism of CrVI Removal in Fe0-based filtration systems. Water (Switzerland), vol. 10, no. 5. doi: 10.3390/w10050651 [ Links ]
Gülcan, M.F., Karahan, B.D., Gürmen, S. 2020. Leaching of iron and chromium from an indigenous ferro chromium alloy via a rotary evaporator: optimum conditions determination and kinetic analysis. Journal of Materials Research and Technology, vol. 9, no. 6, pp. 14103-14115. doi: 10.1016/j.jmrt.2020.09.133 [ Links ]
Hariharan, A.V.L.N.S.H., Murali Krishna, D. 2013. Recovery of chromium from ferrochrome slag. Journal of Chemical and Pharmaceutical Research, vol. 5, no. 5, pp. 250-252. doi: 10.15680/iiirset.2015.0405041 [ Links ]
Holappa, L., Xiao, Y. 2004. Slags in ferroalloys production-review of present knowledge Chromium-oxygen system, (January), pp. 25-28. https://hdl.handle.net/10520/ATA0038223X-2944 [ Links ]
Horckmans, L. 2019. Multi-analytical characterization of slags to determine the chromium concentration for a possible reextraction. Minerals, vol. 9, no. 10, pp. 1-14. doi: 10.3390/min9100646 [ Links ]
Kanari, N. 2020. Reactivity of low-grade chromite concentrates towards chlorinating atmospheres. Materials, vol. 13, no. 20, pp. 1-18. doi: 10.3390/ma13204470 [ Links ]
Kim, E. 2016. New method for selective Cr recovery from stainless steel slag by NaOCl assisted alkaline leaching and consecutive BaCrO4 precipitation. Chemical Engineering Journal. Elsevier B.V., vol. 295, no. 2016, pp. 542-551. doi: 10.1016/j.cej.2016.03.073 [ Links ]
Kocaoba, S., Akcin, G. 2004. Chromium (III) removal from wastewaters by a weakly acidic resin containing carboxylic groups. Adsorption Science and Technology, vol. 22, no. 5, pp. 401-410. doi: 10.1260/0263617042863020 [ Links ]
Lokothwayo, R.B. 2007. Hexavalent chromium analysis, reduction and stabilization in cement and concrete, p. 142. http://hdl.handle.net/10539/4649 [ Links ]
Mane, C.P. 2016. Hexavalent chromium recovery by liquid-liquid extraction with 2-octylaminopyridine from acidic chloride media and its sequential separation from other heavy toxic metal ions. Arabian Journal of Chemistry, vol. 9, pp. S1420-S1427. King Saud University. doi: 10.1016/j.arabic.2012.03.021 [ Links ]
Pacific, A., America, S., Forecasts, S. 2020. Regional Insights, (Cr VI), pp. 2020-2025. RI 43079 www.reportinsights.com/industry-forecasts/chromium-salt-market-researchreport43079 [ Links ]
Pfaff, G. 2022. Chromium oxide pigments. Physical Sciences Reviews, vol. 7, no. 2, pp. 103-108. doi: 10.1515/psr-2020-0159 [ Links ]
Ramakrishnaiah, C.R., PrathimaB. 2011. Hexavalent Chromium Removal by Chemical Precipitation Method: A Comparative Study. International Journal of Environmental Research and Development, pp. 41-49. [ Links ]
Ramakrishnaiah, C.R., Prathima, B. 2012. Hexavalent Chromium Removal From Industrial Watsewater By Chemical Precipitation Method. International Journal of Engineering Research and Applications, vol. 2, no. 2, pp. 599-603. [ Links ]
Sahu, N., Biswas, A., Kapure, G.U. 2016. A Short Review on Utilization of Ferrochromium Slag. Mineral Processing and Extractive Metallurgy Review, vol. 37, no. 4, pp. 211-219. doi: 10.1080/08827508.2016.1168415 [ Links ]
Shen, H., Forssberg, E. 2003. An overview of recovery of metals from slags. Waste Management, vol. 23, no. 10, pp. 933-949. doi: 10.1016/S0956-053X(02)00164-2 [ Links ]
Weng, C.H. 1994. Chromium leaching behavior in soil derived from chromite ore processing waste. Science of The Total Environment. Elsevier, vol. 154, no. 1, pp. 71-86. doi: 10.1016/0048-9697(94)90615-7 [ Links ]
Wiryawan, A. 2018. '120-499-2-Pb'. Journal of Environmental Engineering and Sustainable Technology, vol. 5, no. 01, pp. 37-46. [ Links ]
Zhao, Q. 2014. Sulfuric acid leaching of South African chromite. Part 1: Study on leaching behavior. International Journal of Mineral Processing, vol. 130, pp. 95-101. Elsevier B.V. doi: 10.1016/i.minpro.2014.04.002 [ Links ]
 Correspondence:
Correspondence:
F. Ntuli
Email: ntulif@biust.acbw
Received: 19 Aug. 2024
Published: December 2024













