Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Vision and Eye Health
On-line version ISSN 2410-1516Print version ISSN 2413-3183
AVEH vol.82 n.1 Cape Town 2023
https://doi.org/10.4102/aveh.v82i1.775
ORIGINAL RESEARCH
Environmental impact and end-of-life options of disposed polymeric spectacle and contact lenses
Rayishnee PillayI; Rekha HansrajI; Nishanee RampersadI; Ajay BissessurII
IDiscipline of Optometry, School of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IISchool of Chemistry and Physics, College of Agriculture, Engineering and Science, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: Global population growth and ageing are factors that contribute towards an anticipated increase in the usage of spectacles and contact lenses for vision correction. The subsequent disposal of polymeric vision corrective devices currently, has uncertain environmental impacts.
AIM: The purpose of this study was to explore potential environmental impacts and end-of-life (EOL) pathways of a sample of polymeric spectacle lenses and through the use of analytical chemistry processes.
SETTING: Laboratory analysis of ophthalmic lenses.
METHODS: Inductively coupled plasma-optical emission spectroscopy (ICP-OES), elemental analysis and calorific value investigations were conducted on a sample of spectacle lenses and contact lenses.
RESULTS: Metal ion analysis by ICP-OES confirmed the presence of manganese in all the lenses and chromium in two of the 13 contact lenses. All of the lenses had over 42% carbon while calorific values of up to 32.40 MJ/kg and 23.31 MJ/kg were found in the spectacle lenses and contact lenses, respectively.
CONCLUSION: Further investigation is required regarding the presence of chromium in two of the contact lenses. In general, lenses are likely to remain as solid waste in landfills depending on the disposal conditions. Considering their calorific values, lenses would be useful in incineration with energy recovery processes however the suggested ideal EOL route would be the implementation of lens recycling, through non-toxic and green chemical processes, to retain material value and promote a circular economy.
CONTRIBUTION: This study provides new information on the environmental consequences of current modes of lens disposal and suggests EOL alternatives thereof.
Keywords: environmental biocompatibility; spectacle lenses; contact lenses; ICP-OES; elemental analysis; calorific values; lens disposal.
Introduction
It is estimated that 2.2 billion people worldwide have vision impairment that affects their quality of life, 42% of whom have unaddressed refractive error or presbyopia that may be corrected with spectacles, contact lenses (CLs) or refractive surgery.1 Approximately 64% of the global adult population wore spectacles2 in 2010 and a reported 2% wore CLs.3 With a growing, ageing population entering presbyopia and predictions of a 50% global prevalence of myopia4 by 2050, it is anticipated that there will be an increase in the use of spectacles and CLs. Consequently, there may be an increase in plastic waste from discarded spectacles and CLs as these are replaced over a limited lifespan of lens wear.
Spectacle wearers tend to replace their eyewear on average every 2 years5 while CL wearers can opt for other modalities, including daily, 2-weekly, monthly, or annual replacement. End-of-life (EOL) options for spectacles are often restricted to landfill disposal.5 Spectacle frame materials include metals, such as titanium, stainless steel or metal alloys, or plastics such as cellulose acetate, propionate, polyamide and polycarbonate. Upon disposal, these materials typically exhibit poor degradation capacity and may remain as solid waste for an indeterminate period under natural conditions.5 Some frame materials may contain heavy metals such as lead and chromium, which could leach into and contaminate the surrounding environment.5 Although recent spectacle frame material developments include the introduction of bio-acetate and hexetate, which are biodegradable and marketed as eco-friendly,6 these materials currently have an underdeveloped market share representation.
Contact lenses may be discarded into the waste bin or flushed down the sink or toilet hence its EOL terminus may have environmental impacts. A study in the United States found that nearly 21% of CL wearers flushed their CLs, resulting in a dry mass volume of over 42 tonnes of CLs that entered wastewater streams.7 Medical devices should comply with the International Organization for Standardization biocompatibility regulations and the disposal of certain medical devices, which are contaminated after use, should follow designated protocols.8 However, there are currently no regulated protocols for CL disposal and the environmental impact of lens disposal is still uncertain.
Research on plastic waste disposal has been conducted in several industries such as food and packaging but there are limited studies on the environmental consequences of the disposal of polymeric spectacles and CLs.5,7 Spectacle lenses and CLs have a unique chemistry, comprising polymers of a hydrocarbon backbone incorporating various elements such as oxygen, nitrogen, sulphur, fluorine, silicone, chlorine and phosphorous.9 Polymerisation techniques and the use of additives imparts properties of optical transparency, durability, chemical resistance, ultraviolet (UV) wavelength absorption and thermal stability to the lenses.10 Lenses may also be classified as thermoset or thermoplastic, with the former having a densely cross-linked polymer network that is irreversibly bound after the curing process.9 To explore the potential environmental impact of lens disposal, it is essential to understand the constituent components of the lens materials.
Characteristics of spectacle lens materials
Spectacle lenses are synthesised by the polymerisation and cross-linking of a unique resin formulation.11 Several types of polymeric materials may be used to manufacture spectacle lenses, including acrylics, polythiourethanes, polycarbonates, polystyrenes and polysulfones.12,13 The most widely used resin for lenses in the correction of low ametropia is a 1.49 index lens made from diethylene glycol bis(allyl carbonate) resin11 and marketed as CR-39®. Whereas, for the correction of moderate to high ametropia, high refractive index materials, of 1.6 and higher, may be synthesised using polyurethane, to generate thinner lenses.12
Various additives such as mould release agents, UV absorbers, tints, photochromic dyes, optical brighteners, light and thermal stabilisers, plasticisers, and antioxidants may be incorporated into the monomeric starter materials to attain the required material properties.11,12,13 Furthermore, inorganic surface coatings, such as scratch resistant (or hard coat) and antireflection coatings, may be added to the lens substrate. The scratch resistant coatings are often silicone-based resins, such as silicon dioxide (SiO2), cured in the presence of heat or UV, whereas antireflection coatings are applied in multistack formation with alternating low and high index materials, such as SiO2 and titanium dioxide.14
Characteristics of contact lens materials
Contact lenses may be broadly categorised into hard CLs (water content < 10% by weight) or soft CLs (water content > 10% by weight).15 Hard CLs are synthesised by polymerising methyl methacrylate with a free radical initiator into poly(methyl methacrylate) (PMMA) buttons, which are then lathe-cut and polished to the required refractive and fit parameters.16 Gas permeable CLs, such as silicone acrylate have silicone while fluorosilicone acrylate lenses have fluorine and styrene incorporated onto the methyl acrylate monomer to improve oxygen permeability and improve wearer comfort, respectively.17
Hydrogel CLs are produced by polymerising 2-hydroxyethyl methacrylate monomer with a cross-linker such as ethylene glycol dimethacrylate, using either a thermal or UV initiator.16 The addition of silicone to the hydrogel monomers resulted in the creation of silicone hydrogel (SiHy) lens materials.16 Although silicone has an inherent oxygen permeability, it is also hydrophobic thus causing wearer discomfort.16 Hydrophilic properties were consequently imparted by either a gas plasma treatment on the lens surfaces, the use of internal wetting agents or semi-interpenetrating polymer networks, or by incorporating long chain hydrophilic macromers.16,18,19 Additives such as UV absorbers, photochromic dyes and pigments may also be integrated into the monomer.20
Constituent components of spectacle and CL materials are closely guarded proprietary information. Patent literature of the lenses indicates the use of organometallic catalysts comprising mercury or lead compounds to initiate the polymerisation stage21 while heat stabilisers may contain cadmium and lead.22 The tints and dyes used in the lenses are metal-based pigments, which may contain cadmium, chromium, lead or manganese pigments23 while photochromic components may include mercury dithizonates.24
Each type of lens has a unique chemistry, depending on the presence of additives, coatings and tints, and therefore they may have a variable and indeterminate environmental effect upon lens disposal. Hence, the purpose of this study was to explore potential environmental impacts and EOL pathways of a sample of spectacle lenses and CLs through the use of inductively coupled plasma-optical emission spectroscopy (ICP-OES), elemental analysis and calorific value (CV) determination.
Research methods and design
This exploratory study used an experimental design to investigate the potential environmental impacts of polymeric spectacle lenses and CLs upon disposal.
Study population and sampling
Various monomers and additives are used in the manufacture of spectacle lenses therefore a range of lens samples was analysed to investigate potential environmental impacts of lenses. A selection of 11 spectacle lenses and 13 CLs were sourced from lens distributors and optometrists based in the KwaZulu-Natal province of South Africa. This included lenses with thermoset and thermoplastic properties ranging from uncoated, coated and tinted, as well as ancillary lenses such as ready-made reading spectacles, dummy lenses from spectacle frames and 3D polarising lenses, which are used to view 3D movies (Table 1). The CLs were selected from hard, gas permeable, hydrogel and SiHy materials (Table 2). The soft CLs are labelled according to the non-proprietary name allocated by the United States Adopted Names Council.
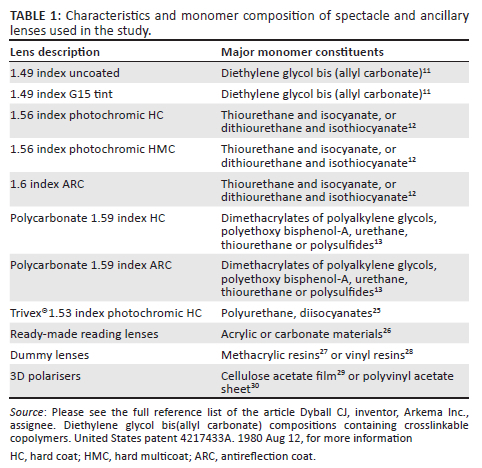
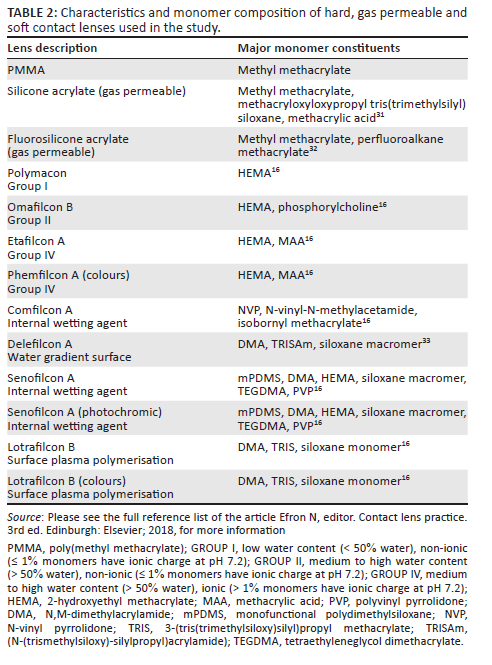
The chemical composition of lenses is proprietary information; therefore, all material constituents and mass fractions thereof are not publicly available. The tests were chosen to investigate the presence of metal ions that may be an environmental contaminant upon lens disposal, to establish the elemental characteristics of the lenses as well as to consider potential EOL options for the lenses.
Data collection and analysis
Metal ion quantification of lenses by inductively coupled plasma-optical emission spectroscopy
Various spectroscopic methods can be used to determine the presence of metal ions, including atomic absorption, graphite furnace atomic absorption, ICP-OES, and ICP-mass spectrometry (MS).34,35 The detection limits of ICP-OES is comparable to most optical spectral techniques,34 and furthermore, it is useful in both qualitative and quantitative analyses and in determining the environmental safety of water, soil and other solid wastes.36 Therefore, ICP-OES was used to investigate the presence and levels of metal ions contained in the sample of lenses. During this method, the prepared lenses were exposed to radio frequency-induced argon plasma and energised to high temperatures.37 The resultant photon emissions with characteristic energies or wavelengths were used to identify the presence of metal ions in the samples.37
Laboratory quality assurance processes were maintained throughout the investigations. Microwave digestion of the lenses was processed to account for the matrix effect and ensure preconcentration of the analyte ions. In order to simulate typical disposal conditions, the soft CLs were rinsed in a multipurpose lens care solution and dehydrated for 14 days prior to digestion and subsequent analysis. The hard CLs in button form were crushed while the soft CLs were proportioned into area dimensions of approximately 16 mm2 (4 mm × 4 mm) using sterilised stainless steel scissors or blades. Approximately 10 mg of sample, digested in concentrated nitric acid, was subjected to microwave digestion in a CEM MARS 6 microwave system using the following conditions:
•Temperature ramp to 180 °C in 15 min
•Holding time at 180 °C for 15 min
•Cooling down for 20 min.
Digested samples were filtered using a 0.45 µm filter and diluted to 100 mL in grade A volumetric flasks. Intensity emissions of samples were scanned by ICP-OES using a PerkinElmer Optima 5300 DV Spectrometer against multielement standards (within working range from 0.1 ppm to 10 ppm) of chromium, manganese, cadmium, mercury and lead.
Percentage composition of carbon, hydrogen, nitrogen and sulphur in the lenses by elemental analysis
Elemental analysis was conducted to determine the percentage of carbon, hydrogen, nitrogen and sulphur in the lenses. The principle of elemental analysis encompasses the combustion of carbon, hydrogen, nitrogen and sulphur to carbon dioxide (CO2), sulphur dioxide (SO2), nitric oxide (NO), nitrogen dioxide (NO2) and water, which is then quantified.38
Knowledge of mass fraction of combustible elements may help to predict CV of the samples,39 as well as provide an indication of the elements that may be released upon lens decomposition. Elemental analysis was conducted using an Elementar vario EL cube Elemental Analyzer. The samples were dried at 105°C for 10 h and approximately 4 mg of sample was subjected to combustion during elemental analysis.
Calorific value determination of spectacle and contact lenses
The CV, or heats of combustion, of a sample refers to the heat or energy released when a mass of the sample is ignited in oxygen in an enclosed unit of constant volume.40 A DryCal modular calorimeter was used and instrument calibration was conducted using benzoic acid. The average weight of the spectacle lenses and CLs used in the investigation were 200 mg and 20 mg, respectively.
Ethical considerations
Ethical approval was received from the Humanities and Social Sciences Research Ethics Committee at the University of KwaZulu-Natal (Reference: HSS/1649/018D). The study involved laboratory analysis of a sample of lenses at the School of Chemistry at the University of KwaZulu-Natal and no further permissions were required.
Results
Metal ion analysis
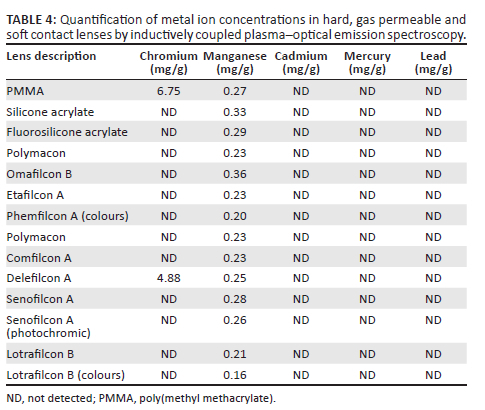
Lens samples were subjected to strong acid digestion (> 6M) and subsequent quantification by ICP-OES. The results thereof (Table 3 and Table 4) indicated the general absence of the investigated metal ions with two notable exceptions. Chromium was detected in the PMMA and Delefilcon A CLs (Table 4) while manganese was detected in all the lenses (Table 3 and Table 4). Cadmium, mercury and lead were absent in the lenses.

Carbon, hydrogen, nitrogen and sulphur elemental analysis of lenses
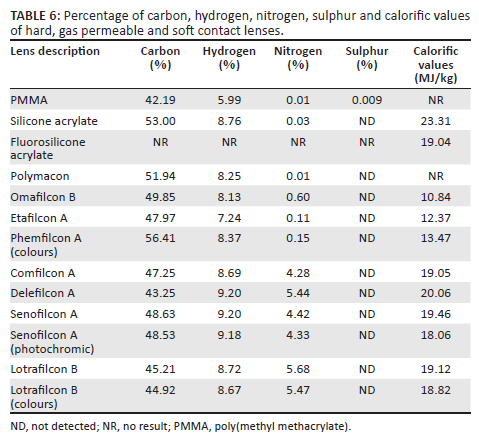
The elemental analysis results (Table 5 and Table 6) revealed that all of the lenses under investigation had over 42% of carbon, with the thermoplastic polycarbonate and Trivex® spectacle lenses having the highest percentage ranging from 63.48% to 70.52% (Table 5). There were nominal amounts of hydrogen in the lenses, ranging from 4.88% to 9.20% (Table 5 and Table 6) with the soft CLs having the highest percentage of hydrogen overall. There were varying levels of nitrogen in the lenses, ranging from none in the 1.49 index lenses to low in the polycarbonate lenses (0.04% - 0.05%) (Table 5). The 1.6 index and Trivex® lens had the highest nitrogen content of 6.75% and 8.22%, respectively (Table 5). Of the CLs, the SiHy lenses had the highest nitrogen percentage ranging between 4.28% and 5.68% (Table 6). All of the spectacle lenses except the Trivex® lens contained sulphur ranging from 0.005% in the uncoated 1.49 index lens to 19.05% in the 1.6 index lens (Table 5). With respect to CLs, the PMMA lens had sulphur of 0.009% while the soft CLs had none (Table 6).

Calorific values of spectacle and contact lenses
Of the thermoset spectacle lenses, the uncoated 1.49 index lens had a CV of 20.74 MJ/kg, while the lenses that contained coatings and tints had higher CVs, ranging from 21.07 MJ/kg to 26.94 MJ/kg (Table 5). The thermoplastic lenses had the highest CV, ranging from 30.29 MJ/kg to 32.40 MJ/kg. Findings were unobtainable for the PMMA and Polymacon CLs. Overall, the CL samples had slightly lower CVs, ranging from 10.84 MJ/kg to 23.31 MJ/kg, as compared with the spectacle lenses.
Discussion
The findings of this study with respect to lens disposal practices is discussed, firstly with disposal in landfills or soil and wastewater streams, secondly by EOL options for lenses in the form of incineration and recycling.
Metal ions in the lenses
Metal ions are common components of feedstock material in lens manufacture and may be used as catalysts to initiate polymerisation processes.39 It is therefore possible that residual unreacted monomers or cross-linking agents may be present in the finished lens substrate which may leach, thus posing a threat as a possible environmental contaminant.41,42 The results from ICP-OES analysis showed the presence of chromium in the PMMA and Delefilcon A CLs (Table 4) and manganese in all the lenses (Table 3 and Table 4). Chromium compounds may be found in the Earth's crust, drinking water or from several industries including metal alloys, electroplating, stainless steel, cement and welding processes, and in the manufacture of pigments.43,44,45 It is commonly found in a trivalent (III) or hexavalent (VI) state. Trivalent chromium is an essential element and considered non-toxic while hexavalent chromium, because of its solubility, is able to diffuse through cell membranes and cause toxic effects.43 Chromium (VI) is a known carcinogen and prolonged exposure thereto can result in renal, liver or neurological dysfunction.44 Occupational Safety and Health Administration permissible exposure limit (OSHA PEL) expressed as a time-weighted average for an 8-h day is 5 µg/m3 for airborne chromium (VI) and is 500 µg/m3 for chromium (III) contamination46 while the Environmental Protection Agency (EPA) has maximum contaminant levels (MCL) of 0.10 mg/L for drinking water.47
The presence of chromium in the lens samples could have occurred through sample preparation whereby, use of apparatus, such as chromium-containing stainless-steel scissors, could have transferred into the samples during processing.48 The PMMA sample (Table 4) was obtained as a blue-coloured button form and required crushing during sample preparation. Crushing exposes the inorganic pigments that would ordinarily be bound to the lens matrix. The Delefilcon A CL, also found to contain chromium (Table 4), is a SiHy CL. Literature indicates that the reusable moulds in the manufacture of SiHy CLs may comprise structures that have a layer of chromium, therefore the lenses may have had contact with chromium compounds during the processing stages,49 which could be a reason for the presence of chromium. Furthermore, CLs are synthesised from monomers comprising carboxylic groups or derivatives of acrylates, all of which are able to absorb metal ions, therefore the potential for chromium adsorption during lens synthesis may be impacted by the constituents of the lens material.50 Quantities of chromium used in lens processing stages are not reported in the patent literature. Typically, elements are bound within the lens matrix51 however upon disposal, and based on ambient conditions, it is possible for lenses to fragment thereby creating the potential for environmental toxicity.
Manganese was found in all of the lens samples. This element is present in the Earth's crust45 and is considered an essential nutrient for the natural system although excessive amounts can be toxic.52 Manganese is used extensively in the iron and steel industry, in the manufacture of dry alkaline batteries and glass.45 Manganese is present in drinking water, and is used in water purification and treatment, in the form of potassium permanganate,45 and this may contribute to manganese presence during the processing of lenses. Manganism, or manganese toxicity, is uncommon and presents with symptoms of neurotoxicity, similar to that of Parkinson's disease.53 The OSHA PEL for manganese is 5 mg/m3 and there is no enforceable MCL for manganese in drinking water.54,55 The levels of manganese within the lenses used in this study ranged from 0.16 mg/g to 0.43 mg/g (Table 5 and Table 6) and is considered to be within acceptable limits of OSHA PEL.
The presence of manganese may be attributed to the use of water in the manufacturing and processing stages of both spectacle lenses and CLs.41 Furthermore, it is possible for the CL packing solution to contain manganese sulphide, for antibacterial purposes, which may adsorb onto the CL surface.56 Another possible reason for the presence of these two metal ions in the CLs is described in the patent literature in which manganese and chromium salts may be included in a redox system to initiate polymerisation on the substrate surface to create hydrophilic, non-fouling CL materials.57 Further investigation of these two CLs regarding chromium content is warranted.
Disposal of lenses into landfills
It is possible for additives within disposed plastic items to migrate to its surface or leach depending on ambient conditions.10,42 Lenses with fixed or photochromic tints may have metal-based pigments incorporated into their substrates.23 Generally, organic pigments and soluble colourants have a low tendency to migrate from the substrate while inorganic pigments, including those containing cadmium, chromium and manganese have no migration tendency, unless the substrate is fragmented through weathering or shear forces.58 The environmental condition of the landfill usually determines the migration of additives from the lenses. Acidic conditions promote the release of inorganic additives while high temperatures allow for the release of both organic and inorganic additives.59
Lenses have unique properties that could make them a vector for contamination. These properties include material surface charge, pore size, the presence of additives, cross-links and hydrophobic or hydrophilic groups in the surface coating and within the lens matrix as well as lens size and thickness, all of which could result in adsorption of contaminants from the surrounding environments.50 Landfills may contain various toxins from discarded items, for example, improper disposal of batteries and fluorescent light bulbs, which could result in leaching of cadmium, nickel and mercury into the soil and ground or surface water.43,60 Lenses disposed in such contaminated environments may potentially adsorb these pollutants, depending on their material properties and prevailing soil conditions.7 This would be problematic if these contaminated lenses entered groundwater or wastewater streams.
Lenses that are discarded into bins are typically relegated to solid waste in landfills. Solid waste may be either compacted and allowed to degrade naturally or incinerated to reduce solid waste volumes. The mobility of polymer networks is linked to its melting point. Spectacle lens polymers are either amorphous or may have a high melting temperature, therefore the thermal stability of the lens materials affects its degradation and decomposition properties. Materials with higher thermal stability have poor degradation under natural conditions and are therefore likely to remain as solid waste.61 Thermal analysis techniques such as thermogravimetric analysis and differential scanning calorimetry would be useful in determining thermal stability and establishing EOL potential for ophthalmic lenses discarded in landfills. Materials such as polycarbonate and Trivex® have an inherent impact resistance and consequently are difficult to physically fracture with routine wear. Upon disposal these materials may be resistant to breakage. Polycarbonate created from bisphenol A (BPA) is non-biodegradable and can persist in nature for a long period.61 Uncontrolled disposal of polycarbonate products is of concern as strong alkaline or acidic conditions and high temperatures can promote the release of BPA from the polycarbonate material through leaching or hydrolysis.42,62
Upon prolonged dehydration and exposure to shear forces CLs may fragment into microplastic-sized segments and when discarded into landfill or soil, the lenses may be assimilated into the soil matrix through various processes such as bioturbation.63 Polymer presence and persistence in soil has an impact on the soil structure, carbon storage, microbial functioning, and soil water holding capacity, thus affecting the inherent biophysical properties of the soil.63 The presence of carbon, hydrogen, nitrogen and sulphur in the disposed lenses may pose as both a benefit and threat to ambient soils. Essential elements for plant growth include the macronutrients, carbon, hydrogen, oxygen, which are provided by air and water.64 Elemental analysis of the investigated lenses revealed carbon levels over 42% and hydrogen of up to 9.2%, which would not pose an environmental threat in the surrounding soil.
Soil-derived nutrients include nitrogen, sulphur and manganese, among others.64 Of the spectacle lenses, the Trivex® lenses had the highest nitrogen percentage of 8.22% while the SiHy CL had the highest percentage of nitrogen of all the CLs under investigation. Plants require nitrogen for growth and there is uptake of nitrogen from soils as ammonium or nitrates.64 High concentration of nitrates (> 10 mg/L) are linked to groundwater contamination and consequent adverse health effects.65 The 1.6 index lens contained the highest percent of sulphur (19.05%) and a nominal amount was found in the PMMA lens (0.009%) while no sulphur was detected in the soft CLs (Table 5). Although sulphur is a soil macronutrient, excessive amounts could be toxic.64 Plant uptake of sulphur is in the form of sulphates and high concentrations of soluble sulphates results in soil toxicity.64
It is noteworthy that lenses that are embedded in soils are unlikely to reach decomposition temperatures through natural processes and therefore the essential elements within the lens will be unavailable to nourish the surrounding soils unless the lenses are fragmented. Hydrogels are being researched for remediation of degraded soils, as their ability to absorb and retain water has been shown to promote plant growth in semi-arid areas.66 Further investigation using CL materials are needed to determine if used CLs could play a role as an EOL remediation option in the agricultural sector, as well as the potential for leaching of elements from ophthalmic lenses upon disposal conditions.
Disposal of contact lenses into aquatic systems
A study in the United States reported that approximately 21% of CL wearers tend to flush their CLs into the wastewater system and that hydrogel CLs are persistent in wastewater treatment processes, potentially fragmenting into microplastic-sized particles.7 Buoyant hydrogel fragments pose an ingestion risk for marine life, they may cause fatal false satiation, and there is potential for uptake into the food chain via fish to humans.67 Plastics suspended in the marine environment are a vector for contaminants as they may also adsorb trace metals.68 The disposed CLs may either sink to sediment level or float into either a water treatment and processing system or the ocean, thus increasing the potential range of contamination.
An additional consideration regarding the environmental impact of disposed lenses is biofilm formation on the lens substrate. This refers to a microorganism aggregation on both biological and non-biological surfaces that are immersed in or surrounded by an aqueous medium.69 These microorganisms may include bacteria, algae, fungi, among others, that adhere to each other and the solid substrate. Disposed lenses are vulnerable to biofilm formation by virtue of the surface coatings, typically hydrophobic for spectacle lenses and hydrophilic for CLs.70 Biofilm formation can impact the buoyancy of disposed CLs and lead to sinking and sedimentation.71 It may also alter the lens surface structure and ionic charge, thus affecting chemical functional groups in the lens material and impacting on its ability to adsorb pollutants from the aqueous environment.71 Conversely, some biofilm microbial species can be useful as they may be a source of bacteria that can degrade microplastic-sized polymers.71 Hydrogels have been in the spotlight for possible use in remediation of heavy metals, dyes and toxic elements from polluted waters.72 A study investigating the diffusion of copper and manganese within hydrogel CLs found that the water content and ionic surface charge were most predictive of ion diffusion,50 thus indicating that the diffusion properties of the different types of lenses may vary if they are to be used for such remediation purposes.
Incineration of lenses
Lenses that are discarded in landfills are vulnerable to ambient conditions of the landfill site. Some municipal landfill sites may conduct open burning of waste in an attempt to reduce waste volumes, therefore lenses disposed under these conditions would be subject to combustion.73 Furthermore, landfill waste, reported to generate CVs between 10 MJ/kg and 21 MJ/kg, may be used for energy recovery processes.73 It is therefore necessary to establish the implications and potential energy value of combusted lenses.
Often plastics that are difficult or uneconomical to recycle are incinerated with or without energy recovery.10 The CVs of the investigated lenses were significant when compared with coal, which is widely used in energy generation. Coal has elemental mass fractions of carbon, hydrogen, nitrogen and sulphur of 72.50%, 5.60%, 1.30% and 0.94%, respectively, and a CV of 27.6 MJ/kg.74 The findings in this study indicate that the spectacle lenses, in particular those with coatings and tints, as well as the thermoplastic materials (polycarbonate and Trivex®) have higher combustion capacities that would be useful in energy recovery processes during incineration. The thermoset lenses are resistant to natural and solvent degradation9 and are a challenge to recycle therefore incineration of these lenses may be a preferred option instead of landfilling. The relatively high CV findings in this study, in comparison to that of coal, suggests that the lenses could be used successfully in this type of energy recovery, however the economics and costs of incinerator facilities requires consistently large volumes of lenses to be profitable. This would entail a dedicated collective effort by optometrists and other eye care personnel of unwanted lenses that would then need to be redirected to such an incinerator facility.
A major drawback of incineration is the consequent release of gaseous emissions, persistent organic pollutants and particulate matter.75 Elemental analysis of the investigated lenses (Table 5 and Table 6) revealed the presence of carbon, nitrogen and sulphur, thus indicating the potential release of CO2, NO2, NO and SO2 upon lens combustion. Typically, during thermal degradation, polymers that contain nitrogen can emit hydrogen cyanide and nitrogen oxides76 while those containing sulphur can emit SO2 or trioxides or may remain in the ash as a by-product of combustion.74 These emissions and ash by-products have an adverse impact on the environment and human health therefore there are strict guidelines controlling waste incineration.10 However, modern incinerators have added features to ensure environmental safety compliance, including scrubbers to neutralise acidic gases, activated carbon to adsorb heavy metals and organic pollutants, reduction systems to remove nitrous oxides, and filter bags for particulate matter.77 An additional consideration is that incineration involves material destruction and does not contribute to the circular economy principle of recycling to maximum material value.10 Furthermore, if ignited in open landfill fires, the CVs of the lenses (Table 5 and Table 6) suggest that they are potential sources of global warming, especially the thermoplastic polycarbonate and Trivex® lenses containing nitrogen and sulphur, which have CVs ranging up to 32.40 MJ/kg.
Recycling of lenses
Some non-profit and aid organisations collected unwanted spectacles for redistribution to individuals who were unable to afford them. Although these were referred to as 'recycling programmes', this was a misnomer as it was not a traditional materials recycling measure. Some of these recycling programmes had a low proportion of useable spectacles and a lack of uptake because of poor quality, varying styles and inadequate refractive correction, resulting in most donated spectacles eventually being disposed as waste.78 This type of programme has been halted for various reasons, including low cost-effectiveness, poor quality of donated spectacles and environmental issues because of unusable frames being discarded.78 A traditional recycling programme has been initiated by an organisation in the United States that collects spectacle lenses, which is repurposed to make safety spectacles and helmet shields.79 With respect to CL recycling, one organisation, to date, namely Terracycle, is known to collect and recycle all CL waste and has programmes in the United States, United Kingdom and Sweden.80 In regions that do not have these recycling options, the disposal routes of CL waste are likely to be in landfill or wastewater streams.
Recycling options include mechanical recycling, in which materials are crushed, extruded and reused for a similar or new application.81 The lenses with thermoset properties are a challenge for mechanical recycling. Mechanical recycling may result in a lower quality of recyclate compared with the original material,81 depending on the level of contamination of the recycled material and on whether it is post-industrial or post-consumer waste. Chemical recycling is an alternative that includes pyrolysis or gasification, whereby plastics are depolymerised into monomeric products that can be used for combustion or creation of new polymers.81 Mechanical recycling is also not preferred for BPA polycarbonate materials as re-extrusion may perpetuate BPA, whereas chemical recycling thereof, using methanolysis, hydrolysis or aminolysis with supercritical solvents, creates depolymerised feedstock material that can be further processed into new polymers.82
Prospects for end-of-life of lens materials
A circular economy favours recycling to retain material value and reduce the need for virgin fossil fuels, as well as to promote natural systems, such as composting to preserve soils.10 With respect to spectacle lenses and CLs, this would entail the redesign of lens materials and associated products and packaging to allow for increased volumes of recycling or material recovery. Thermoset lenses are technically recyclable. The lack of homogeneity in lens materials is a challenge to recycling as the diverse lens substrates, with various inorganic coatings and tints, may be immiscible. It is not possible to sort and separate lenses according to its material constituents by sight, neither are there identifiable markings on the fitted lenses to indicate if they are CR-39®, polyurethane or polycarbonate materials. It may be suggested that, similar to the engravings on progressive addition lenses, manufacturers should engrave a code indicating the lens material on the lens periphery to help with the identification and sorting at EOL for recycling.
The introduction of bioacetate materials for use in spectacle frames is welcomed and the widespread use thereof will promote sustainability.6 Research has focused on the use of biodegradable materials to synthesise thermoset spectacle lenses83 and dummy lenses27 as well as the use of soy protein from the soybean plant for hydrogels.84 Such products would be customised to biodegrade upon specific conditions. There is a renewable supply of corn, sugarcane and soybean and use of these materials would reduce the demand on fossil fuels. Although there are currently some challenges in the manufacture of CLs from these materials regarding material hydrophobicity and poor mechanical properties, once this is overcome, the lower costs, ease of processing, biocompatibility and biodegradability of this material appears promising.84
Limitations of the study
There are many ophthalmic lens manufacturers that produce lenses of varying polymeric materials, additives, coatings and tints. This study was limited to a sample of 24 ophthalmic lenses that are commercially available in South Africa, and the reported study findings may vary for other types of ophthalmic lenses not utilised in this study.
Conclusion
This study explored the potential for environmental toxicity upon lens disposal and evaluated EOL options of a sample of spectacle lenses and CLs. The levels of chromium detected in the PMMA and Delefilcon A lenses were above that recommended by the Occupational Safety and Health Administration (OSHA), and this warrants further investigation, potentially using ICP-mass spectrometry or atomic absorption spectroscopy. Although manganese was found in all of the investigated lenses, this would not pose an environmental threat upon lens disposal. In general, the lenses under investigation, aside from the PMMA and Delefilcon A lenses, are likely to remain as solid waste under natural landfill conditions without potential adverse environmental effects. Comparison of the various lenses in the study sample indicated higher CVs for those with tints and coatings. Furthermore, the high CVs, comparative to coal, indicates that the unwanted lenses would be useful in energy recovery facilities. However, elemental analysis indicated the presence of nitrogen and sulphur in some lenses, therefore if the lenses are incinerated in facilities with non-adherence to environmental safety protocols or if burned in open landfill fires then there is a risk of release of noxious gaseous elements and threat of air pollution. A further consideration is that incineration is not a preferred option in the circular economy model as materials are removed from circulation. Therefore, chemical recycling may be an ideal EOL option for spectacle lenses and CLs, however this is more expensive and would need to be balanced against economies of cost.
Acknowledgements
R.P. is grateful to the College of Health Sciences at the University of KwaZulu-Natal for a research scholarship. The authors acknowledge the kind assistance of Unathi Bongoza and Adela Madaree, from the School of Chemistry and Physics at UKZN, with the elemental analysis and ICP-OES testing, respectively.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
All authors were involved in the conceptualisation and methodology of the article. R.P. completed the literature search, investigation and drafted the research article. R.H., N.R. and A.B. were involved in review and editing of the article to the final version.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability
The data that support the findings of this study are available from the corresponding author, R.P., upon reasonable request.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to vision 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet. 2021;9(2):144-160. https://doi.org/10.1016/S2214-109X(20)30489-7 [ Links ]
2.The Vision Council. Annual report 2015 [homepage on the Internet]. The Vision Council; 2015 [cited 2017 Nov 15]. Available from: https://www.thevisioncouncil.org/annualreport.html [ Links ]
3.Nichols JJ, Willcox MDP, Bron AJ, et al. The TFOS international workshop on contact lens discomfort: Executive summary. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS7-TFOS13. https://doi.org/10.1167/iovs.13-13212 [ Links ]
4.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042. https://doi.org/10.1016/j.ophtha.2016.01.006 [ Links ]
5.Hansraj R, Govender B, Joosab M, Magubane S, Rawat Z, Bissessur A. Spectacle frames: Disposal practices, biodegradability and biocompatibility - A pilot study. Afr Vis Eye Health. 2021;80(1):a621. https://doi.org/10.4102/aveh.v80i1.621 [ Links ]
6.Opto-Réseau. Three materials used to produce eco-friendly glasses [homepage on the Internet]. Opto-Réseau; 2019 [cited 2021 Nov 13]. Available from: https://www.opto-reseau.com/en/trends/articles/3-materials-used-to-produce-eco-friendly-glasses/ [ Links ]
7.Rolsky C, Kelkar VP, Halden RU. Nationwide mass inventory and degradation assessment of plastic contact lenses in US wastewater. Environ Sci Technol. 2020;54:12102. https://doi.org/10.1021/acs.est.0c03121 [ Links ]
8.World Health Organization. WHO Global model regulatory framework for medical devices including in vitro diagnostic medical devices. Geneva: World Health Organization; 2017. [ Links ]
9.Nicholson JW. The chemistry of polymers. 3rd ed. Cambridge, MA: RCS Publishing; 2006. [ Links ]
10.World Economic Forum, Ellen MacArthur Foundation, McKinsey and Company. The new plastics economy - Rethinking the future of plastics [homepage on the Internet]. WEF, EMF; 2016 [cited 2019 May 22]. Available from: http://www.ellenmacarthurfoundation.org/publications [ Links ]
11.Dyball CJ, inventor, Arkema Inc., assignee. Diethylene glycol bis(allyl carbonate) compositions containing crosslinkable copolymers. United States patent 4217433A. 1980 Aug 12. [ Links ]
12.Okoroafor MO, Smith RA, Graham MJ, Tabakovic R, Herold RD, inventors; PPG Industries Ohio, Inc., assignee. Method of preparing an optical polymerizate. International Publication No. WO2001036508A1. 2001 May 25. [ Links ]
13.Richard G, Primel O, Yean L, inventors; Essilor International (Compagnie General d'Optique), assignee. Radically polymerizable composition resulting in shock resistant organic lenses. United States patent 7393880 B2. 2008 Jul 01. [ Links ]
14.Samson F. Ophthalmic lens coatings. Surf Coat Tech. 1996;81(1):79-86. https://doi.org/10.1016/0257-8972(95)02532-4 [ Links ]
15.Gasson A, Morris J. The contact lens manual. 3rd ed. Edinburgh: Butterworth-Heinemann; 2003. [ Links ]
16.Efron N, editor. Contact lens practice. 3rd ed. Edinburgh: Elsevier; 2018. [ Links ]
17.Bennett ES, Henry VA, editors. Clinical manual of contact lenses. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014. [ Links ]
18.Lai Y, Yeh M, Li H, Ting W, inventors; Pegavision Corporation, assignee. Silicone hydrogel composition and silicone hydrogel contact lenses made from the composition. United States patent 9046641 B2. 2015 Jun 2. [ Links ]
19.Tran N, Yang M. Synthesis and characterization of silicone contact lenses based on TRIS-DMA-NVP-HEMA hydrogels. Polymers. 2019;11(6):944. https://doi.org/10.3390/polym11060944 [ Links ]
20.Faubl H, inventor; Wesley Jessen Corporation, assignee. UV blocking lenses and material containing benzotriazoles and benzophenones. United States patent 6244707 B1. 2001 Jun 12. [ Links ]
21.Okoroafor M, Smith R, inventors; PPG Industries Ohio Inc, assignee. Photochromic coated high impact resistant articles. United States patent 20030044620A1. 2003 Mar 6. [ Links ]
22.Munier B, Bendell LI. Macro and micro plastics sorb and desorb metals and act as a point source of trace metals to coastal ecosystems. PLoS One. 2018;13(2):e0191759. https://doi.org/10.1371/journal.pone.0191759 [ Links ]
23.Odeh IN, Markanday MN, Van Peer J, et al, inventors; SABIC Global Technologies, assignee. Color changing material. United States patent 20170174983 A1. 2017 Jun 22. [ Links ]
24.Qin X, Sugimura H, Boulineau MS, Moravec TJ, Harris D, inventors; Insight Equity, assignee. Photochromic lens. United States patent 7858001 B2. 2010 Dec 28. [ Links ]
25.Slagel EC, inventor; BAE Systems Simula Inc., assignee. Impact resistant polyurethane and method of manufacture thereof. United States patent 6127505. 2000 Oct 3. [ Links ]
26.Krefman R, inventor; OTC Optics LLC, assignee. Method for minimizing prism in over-the-counter eyeglasses and optical devices. United States patent US20100020286 A1. 2010 Jan 28. [ Links ]
27.Yamanaka K, Kushida K, Ueda T, inventors; Arukee, Toray Ind Inc., assignees. Dummy lens for eyeglass. Japanese patent 2010197761 A. 2010 Sep 9. [ Links ]
28.Seo K, inventor; Hoya Corporation, assignee. Eyeglass frame mounting hole indicating sign sheet, eyeglass frame mounting hole forming guide sheet, and mounting hole forming method for forming eyeglass frame mounting hole in frameless eyeglass lens using these sheets. Japanese patent 3082005 B2. 2000 Aug 28. [ Links ]
29.Kurtz AF, Diehl DR, inventors; IMAX Theatres International Limited, assignee. Stereo viewing device. International Publication WO2016092490 A1. 2016 Jun 16. [ Links ]
30.Kessler D, Matera P, inventors; SOL Grid LLC, assignee. 3D polarized eyewear. United States patent 20140029096 A1. 2014 Jan 30. [ Links ]
31.Ellis EJ, Mager L, inventors; B&L International Holdings Corp, assignee. Dimensionally stable oxygen permeable hard contact lens material and method of manufacture. United States patent 4330383 A. 1982 May 18. [ Links ]
32.Walker J. A clinical and laboratory comparison of the deposition characteristics of both silicone/acrylate and flurosilicone-acrylate lenses. J Br Contact Lens Assoc. 1988;11:83-86. [ Links ]
33.Qiu Y, Pruitt JD, Thekveli SJ, Tucker RC, Nelson J, inventors; Alcon Inc, assignee. Silicone hydrogel lenses with water-rich surfaces. United States patent 8480227 B2. 2013 Jul 9. [ Links ]
34.Skoog DA, Holler FJ, Crouch SR. Principles of instrumental analysis. 7th ed. Boston, MA: Cengage Learning; 2018. [ Links ]
35.Ritter A, Michel E, Schmid M, et al. Interlaboratory test on polymers: Determination of heavy metals in polymer metrices. Polym Test. 2004;23(4):467-474. https://doi.org/10.1016/j.polymertesting.2003.09.001 [ Links ]
36.Thermo Fisher Scientific Inc. Comparison of ICP-OES and ICP-MS for trace element analysis [homepage on the Internet]. Thermo Fisher Scientific Inc; 2022 [cited 2022 Dec 12]. Available from: https://www.thermofisher.com/za/en/home/industrial/environmental/environmental-learning-center/contaminant-analysis-information/metal-analysis/comparison-icp-oes-icp-ms-trace-element-analysis.html [ Links ]
37.Hou X, Amais RS, Jones BT, Donati GL. Inductively coupled plasma optical emission spectrometry. In: Meyers RA, editor. Encyclopedia of analytical chemistry: Applications, theory and instrumentation. Chichester: Wiley Online Library, 2021; p. 9468-9485. [ Links ]
38.Elementar. vario EL cube - CHNS elemental analyzer [homepage on the Internet]. Elementar; 2020 [cited 2021 Sept 28]. Available from: https://www.elementar.com/en/products/organic-elemental-analyzers/vario-el-cube [ Links ]
39.Gnaiger E, Bitterlich G. Proximate biochemical composition and caloric content calculated from elemental CHN analysis: A stoichiometric concept. Oecologia. 1984;62(3):289. https://doi.org/10.1007/BF00384259 [ Links ]
40.Núñez Regueira L. Bomb calorimeter. In: Cleveland CJ, Morris C, editors. Dictionary of energy. 2nd ed. Oxford: Elsevier, 2015; p. 67. [ Links ]
41.Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: A review of patents and commercial products. Eur Polym J. 2015;65:252-267. https://doi.org/10.1016/j.eurpolymj.2014.11.024 [ Links ]
42.Teuten EL, Saquing JM, Knappe DR, et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc Lond B Biol Sci. 2009;364(1526):2027-2045. https://doi.org/10.1098/rstb.2008.0284. [ Links ]
43.Martin S, Griswold W. Environmental science and technology briefs for citizens, Center for Hazardous Substance Research, Kansas State University [homepage on the Internet]. Kansas State University; 2009 [cited 2022 Jan 19]. Available from: https://engg.k-state.edu/chsr/files/chsr/outreach-resources/15HumanHealthEffectsofHeavyMetals.pdf [ Links ]
44.Wang Y, Su H, Gu Y, Song X, Zhao J. Carcinogenicity of chromium and chemoprevention: A brief update. Onco Targets Ther. 2017;10:4065-4079. https://doi.org/10.2147/OTT.S139262 [ Links ]
45.Nordberg GF, Fowler BA, Nordberg M, editors. Handbook on the toxicology of metals. 4th ed. Amsterdam: Elsevier; 2015. [ Links ]
46.Agency for Toxic Substances and Disease Registry. Chromium toxicity [homepage on the Internet]. ATSDR; 2013 [cited 2022 Jan 19]. Available from: https://www.atsdr.cdc.gov/csem/chromium/standards_and_regulations.html [ Links ]
47.U.S. Environmental Protection Agency. Chromium in drinking water [homepage on the Internet]. EPA; 2022 [cited 2022 Jan 19]. Available from: https://www.epa.gov/sdwa/chromium-drinking-water [ Links ]
48.Santonen T, Stockmann-Juvala H, Zitting A. Review on toxicity of stainless steel. Helsinki: Finnish Institute of Occupational Health; 2010. [ Links ]
49.Liu AW, Dietrich B, inventors; Novartis AG, assignee. Molds for making contact lenses, International patent 2015095565A. 2015 Jun 25. [ Links ]
50.Krysztofiak K, Szyczewski A, Kruczyński Z. Investigation of copper and manganese ion diffusion through hydrogel contact lens materials using ESR technique. Pol J Environ Stud. 2014;23(2):363-371. [ Links ]
51.Angelo Green J, Scott Phillips K, Hitchins VM, et al. Material properties that predict preservative uptake for silicone hydrogel contact lenses. Eye Contact Lens. 2012;38(6):350-357. https://doi.org/10.1097/ICL.0b013e318272c470 [ Links ]
52.Millaleo R, Reyes-Díaz M, Ivanov AG, Mora ML, Alberdi M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J Soil Sci Plant Nutr. 2010;10(4):470-481. https://doi.org/10.4067/S0718-95162010000200008 [ Links ]
53.Evans GR, Masullo LN. Manganese toxicity [homepage on the Internet]. FL: StatPearls Publishing; 2021 [cited 2022 Jan 19]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560903/ [ Links ]
54.The National Institute for Occupational Safety and Health. Manganese Compounds and Fume (as Mn) [homepage on the Internet]. CDC; 2019 [cited 2022 Jan 19]. Available from: https://www.cdc.gov/niosh/npg/npgd0379.html [ Links ]
55.Water Quality Association. Manganese [homepage on the Internet]. WQA; 2019 [cited 2022 Jan 19]. Available from: https://www.wqa.org/learn-about-water/water-q-a/manganese [ Links ]
56.Alvarez-Carrigan N, Brown-Skrobot S, Wong Meyers A, Neely F, Pall B, Rathore O, inventors; Johnson and Johnson Vision Care Inc., assignee. Packaging solution for ophthalmic device. European patent 1765425A1. 2007 Mar 28. [ Links ]
57.Zhang Z, Li J, Skinner M, Loose CR, Coury AJ, inventors; Semprus Biosciences Corp, assignee. Redox processes for contact lens modification. International Publication WO2013090813 A1. 2013 Jun 20. [ Links ]
58.Hansen E, Nilsson NH, Lithner D, Lassen C. Hazardous substances in plastic materials. Vejle: COWI and Danish Technological Institute; 2013. [ Links ]
59.Liu P, Zhan X, Wu X, Li J, Wang H, Gao S. Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere. 2019;242(3):25193. https://doi.org/10.1016/j.chemosphere.2019.125193 [ Links ]
60.Department of Environmental Affairs. Inventory of Mercury Releases in South Africa [homepage on the Internet]. Pretoria: AJUA Environmental Consultants and DEA; 2011 [cited 2022 Jan 19]. Available from: https://www.unep.org/resources/report/inventory-mercury-releases-south-africa [ Links ]
61.Artham T, Doble M. Biodegradation of aliphatic and aromatic polycarbonates. Rev Macromol Biosci. 2008;8(1):14-24. https://doi.org/10.1002/mabi.200700106 [ Links ]
62.Chow JT. Environmental assessment for Bisphenol-A and polycarbonate [MSc thesis]. Kansas State University; 2007 [cited 2022 Jan 19]. Available from: https://core.ac.uk/download/pdf/5164464.pdf [ Links ]
63.De Souza Machado AA, Lau CW, Till J, et al. Impacts of microplastics on the soil biophysical environment. Environ Sci Technol. 2018;52(17):9656-9665. https://doi.org/10.1021/acs.est.8b02212 [ Links ]
64.Mahler RL. Nutrients plants require for growth [homepage on the Internet]. University of Idaho, Idaho Agricultural Experiment Station; 2004 [cited 2022 Jan 19]. Available from: https://www.extension.uidaho.edu/publishing/pdf/CIS/CIS1124.pdf [ Links ]
65.Agency for Toxic Substances and Disease Registry. Nitrate/nitrite toxicity [homepage on the Internet]. ATSDR; 2014 [cited 2022 Jan 19]. Available from: https://www.atsdr.cdc.gov/csem/nitrate-nitrite/standards.html [ Links ]
66.Van Tran V, Park D, Lee Y. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ Sci Pollut Res. 2018;25(25):24569-24599. https://doi.org/10.1007/s11356-018-2605-y [ Links ]
67.Cole M, Lindeque P, Halsband C, Galloway TS. Microplastics as contaminants in the marine environment: A review. Mar Pollut Bull. 2011;62(12):2588-2597. https://doi.org/10.1016/j.marpolbul.2011.09.025 [ Links ]
68.Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci. 2016;178:189-195. https://doi.org/10.1016/j.ecss.2015.12.003 [ Links ]
69.Han S, Ji S, Abdullah A, Kim D, Lim H, Lee D. Superhydrophilic nanopillar-structured quartz surfaces for the prevention of biofilm formation in optical devices. Appl Surf Sci. 2018;429:244-252. https://doi.org/10.1016/j.apsusc.2017.07.164 [ Links ]
70.Willcox MDP. Microbial adhesion to silicone hydrogel lenses: A review. Eye Contact Lens. 2013;39(1):61-66. https://doi.org/10.1097/ICL.0b013e318275e284 [ Links ]
71.Tu C, Chen T, Zhoua Q, et al. Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Sci Total Environ. 2020;734:139237. https://doi.org/10.1016/j.scitotenv.2020.139237 [ Links ]
72.Bahram M, Mohseni N, Moghtader M. An introduction to hydrogels and some recent applications. In: Majee SB, editor. Emerging concepts in analysis and applications of hydrogels. London: InTechOpen, 2016; p. 9-38. [ Links ]
73.Cheela VRS, John M, Dubey B. Quantitative determination of energy potential of refuse derived fuel from the waste recovered from Indian landfill. Sustain Environ Res. 2021;31(1):24. https://doi.org/10.1186/s42834-021-00097-5 [ Links ]
74.Krajnc N. Wood fuels handbook. Pristina: Food and Agriculture Organization of the United Nations; 2015. [ Links ]
75.Panda AK, Singh RK, Mishra DK. Thermolysis of waste plastics to liquid fuel: A suitable method for plastic waste management and manufacture of value added products - A world prospective. Renew Sust Energ Rev. 2010;14(1):233-248. https://doi.org/10.1016/j.rser.2009.07.005 [ Links ]
76.Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater. 2018;344:179-199. https://doi.org/10.1016/j.jhazmat.2017.10.014 [ Links ]
77.Lam CHK, Ip AWM, Barford JP, McKay G. Use of incineration MSW ash: A review. Sustainability. 2010;2(7):1943-1968. https://doi.org/10.3390/su2071943 [ Links ]
78.IAPB. Position paper on recycled spectacles [homepage on the Internet]. IAPB; 2014 [cited 2021 Dec 28]. Available from: https://www.iapb.org/wp-content/uploads/2020/10/Position-Paper-on-Recycled-Spectacles.pdf [ Links ]
79.Eyecare Business. Costa unveils plastic lens recycling program [homepage on the Internet]. Eyecare Business; 2019 [cited 2022 Jan 19]. Available from: https://www.eyecarebusiness.com/news/2019/costa-unveils-plastic-lens-recycling-program [ Links ]
80.Terracycle UK. Acuvue®Contact lens recycle programme [homepage on the Internet]. Terracycle UK; 2019 [cited 2022 Jan 19]. Available from: https://www.terracycle.com/en-GB/brigades/acuvue [ Links ]
81.Payne J, McKeown P, Jones MD. A circular economy approach to plastic waste. Polym Degrad Stab. 2019;165:170-181. https://doi.org/10.1016/j.polymdegradstab.2019.05.014 [ Links ]
82.Bhogle CS, Pandit AB. Ultrasound assisted methanolysis of polycarbonate at room temperature. Ultrason Sonochem. 2019:58:104667. https://doi.org/10.1016/j.ultsonch.2019.104667 [ Links ]
83.Batia M, Jesmalani J, Leuti SC, inventors; Novol Inc., assignee. Sorbitol-based crosslinked optical polymer. South Korea patent 20200110355A. 2020 Sept 23. [ Links ]
84.Dorishetty P, Balu R, Sreekumar A, et al. Robust and tunable hybrid hydrogels from photo-cross-linked soy protein isolate and regenerated silk fibroin. ACS Sustain Chem Eng. 2019;7(10):9257-9271. https://doi.org/10.1021/acssuschemeng.9b00147 [ Links ]
 Correspondence:
Correspondence:
Rayishnee Pillay
9300354@stu.ukzn.ac.za
Received: 09 May 2022
Accepted: 16 Jan. 2023
Published: 20 June 2023













