Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.110 n.12 Pretoria Dec. 2020
http://dx.doi.org/10.7196/samj.2020.v110i12.14419
RESEARCH
The 'ins and outs' of colonoscopy at Wits Donald Gordon Medical Centre, South Africa: A practice audit of the outpatient endoscopy unit
C BouterI; P BarrowII, III; D BizosIV, V, VI; B BobatVII; J DevarVIII, IX; N HarranX; C JosephXI, XII; A MahomedXIII, XIV; J OettleXV; J RamosXVI, XVII; M SeabiXVIII, XIX; G KgabageXX; P GaylardXXI; D SurridgeXXII, XXIII; H EtheredgeXXIV, XXV; J FabianXXVI, XXVII; D LutrinXXVIII, XXIX; K KarlssonXXX
IBHSc; Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
IIMB BCh, DCH (SA), FCP (SA), Cert Gastroenterology (SA); Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
IIIMB BCh, DCH (SA), FCP (SA), Cert Gastroenterology (SA); Department of Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVMB BCh, MMed (Surg), FCS (SA); Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
VMB BCh, MMed (Surg), FCS (SA); Department of Surgery, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VIMB BCh, MMed (Surg), FCS (SA); Surgical Gastroenterology Unit, Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
VIIMB ChB, Dip HIV Man (SA), FCP (SA), Cert Gastroenterology (SA); Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
VIIIMB BCh, FCS (SA), Cert Gastroenterology (SA) Surg; Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
IXMB BCh, FCS (SA), Cert Gastroenterology (SA) Surg; Gastroenterology Unit, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
XMB BCh, MMed (Surg), FCS (SA), Cert Gastroenterology (SA) Surg; Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
XIMB BCh, DA (SA), FCS (SA), Cert Gastroenterology (SA); Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
XIIMB BCh, DA (SA), FCS (SA), Cert Gastroenterology (SA); Department of Surgery, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XIIIMB BCh, FCP (SA), Cert Gastroenterology (SA); Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
XIVMB BCh, FCP (SA), Cert Gastroenterology (SA); Department of Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XVBSc Hons, MB BCh, FRCS (Ed); Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
XVIMB BCh, FCS (SA); Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
XVIIMB BCh, FCS (SA); Department of Surgery, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XVIIIMB ChB, FCP (SA), Cert Gastroenterology (SA), MMed (Int Med); Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
XIXMB ChB, FCP (SA), Cert Gastroenterology (SA), MMed (Int Med); Department of Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XXRN; Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
XXIPhD; Data Management and Statistical Analysis (DMSA), Johannesburg, South Africa
XXIIMB ChB, FCS (SA), MMed (Surg), Cert Gastroenterology (SA) Surg; Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
XXIIIMB ChB, FCS (SA), MMed (Surg), Cert Gastroenterology (SA) Surg; Gastroenterology Unit, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
XXIVPhD; Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
XXVPhD; Department of Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XXVIMB BCh, FCP (SA), Cert Nephrology (SA); Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
XXVIIMB BCh, FCP (SA), Cert Nephrology (SA); Department of Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XXVIIIMB BCh, DA (SA); Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
XXIXMB BCh, DA (SA); Department of Surgery, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XXXMB ChB, MRCP (UK), FCP (SA); Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: In South Africa, there are no national guidelines for the conduct or quality assessment of colonoscopy, the gold standard for investigation and diagnosis of bowel pathology
OBJECTIVES: To describe the clinical profile of patients and evaluate the practice of colonoscopy using procedural quality indicators at the Wits Donald Gordon Medical Centre (WDGMC) outpatient endoscopy unit (OEU
METHODS: We conducted a prospective, clinical practice audit of colonoscopies performed on adults (>18 years of age). A total of 1 643 patients were included in the study and variables that were collected enabled the assessment of adequacy of bowel preparation, length of withdrawal time and calculation of caecal intubation rate (CIR), polyp detection rate (PDR) and adenoma detection rate (ADR). We stratified PDR and ADR by sex, age, population group, withdrawal time and bowel preparation. CIR, PDR and ADR estimates were compared between patient groups by the x2 test; Fisher's exact test was used for 2 x 2 tables. A p-value <0.05 was used. Benchmark recommendations by the American Society for Gastrointestinal Endoscopy (ASGE)/American College of Gastroenterology (ACG) Task Force on Colorectal Cancer (CRC) were used in this audit to assess individual endoscopist performance and that of the endoscopy unit as a whole
RESULTS: The mean age of patients was 55.7 (standard deviation (SD) 14.4; range 18 - 91) years, ~60% were female, and the majority (75.5%) were white. Of the outpatients, 77.6% had adequate bowel preparation (ASGE/ACG benchmark >85%). The CIR was 97.0% overall, and screening colonoscopy was 96.3% (ASGE/ACG benchmark >90% overall and >95% for screening colonoscopies). The median withdrawal time for negative-result screening colonoscopies was 5.7 minutes (interquartile range (IQR) 4.2 - 9.3; range 1.1 - 20.6) (ASGE/ACG benchmark > 6minutes), and PDR and ADR were 27.6% and 15.6%, respectively (ASGE/ACG benchmark ADR >25%). We demonstrated a 23.7% increase in PDR and 14.1% increase in ADR between scopes that had mean withdrawal times of >6 minutes and <6 minutes, respectively. Although the number of black Africans in the study was relatively small, our results showed that they have similar ADRs and PDRs to the white population group, contradicting popular belief
CONCLUSIONS: The WDGMC OEU performed reasonably well against the international guidelines, despite some inadequacy in bowel preparation and lower than recommended median withdrawal times on negative-result colonoscopy. Annual auditing of clinical practice and availability of these data in the public domain will become standard of care, making this audit a baseline for longitudinal observation, assessing the impact of interventions, and contributing to the development of local guidelines
Colonoscopy is regularly used for investigation of bowel pathology and has become the gold standard for screening and diagnosis of colorectal cancer (CRC).[1] The procedure has diagnostic and therapeutic benefits, such as direct visualisation of the entire colon and removal of precancerous polyps, which is associated with a lowered risk of CRC.[2] Colonoscopy is a skill-intensive procedure and poses a risk to the patient, even if performed by a trained endoscopist in an appropriate setting.[1] Therefore, there is a need for standardised practice and regular audit of endoscopists to ensure consistent, high-quality care.[3]
Based on the 'adenoma-carcinoma sequence' hypothesis for developing CRC, screening and surveillance colonoscopy aim to detect and remove polyps, particularly adenomatous polyps, with the intention of reducing the incidence of CRC.[4] Therefore, the polyp detection rate (PDR) and adenoma detection rate (ADR) are two key indicators of the quality of endoscopy. Other measurable factors, such as adequate bowel preparation, reaching the caecum or terminal ileum and scope withdrawal time, all affect the clinician's ability to detect polyps and should be scrutinised alongside ADR and PDR.[2,5] Unfortunately, there are no locally derived guidelines or benchmarks for measurement of quality of colonoscopy in South Africa (SA). International guidelines, such as the American Society for Gastrointestinal Endoscopy (ASGE)/American College of Gastroenterology (ACG) Task Force on Colorectal Cancer can be used to benchmark our performance as a unit and that of individual endoscopists.[6]
Wits Donald Gordon Medical Centre (WDGMC) in Johannesburg, SA is part of the University of the Witwatersrand academic teaching hospital complex and was the first private academic hospital in SA. In 2006, a formal outpatient endoscopy unit (OEU) was established that currently hosts 14 endoscopists. All endoscopists have medical or surgical specialist training in endoscopy. In this audit, we aimed to describe the clinical profile of patients undergoing colonoscopy at WDGMC and to evaluate the practice of colonoscopy in the OEU by assessing intraprocedural quality indicators.
Methods
We conducted a prospective, clinical practice audit of colonoscopies performed on adults (>18 years of age) between 1 March 2018 and 28 February 2019 at the WDGMC OEU. Each endoscopist agreed to participate in the audit, and we obtained written informed consent from all patients. The attending nurse collected details of the colonoscopy at the time of the procedure using a standardised data collection sheet (Appendix A: http://samj.org.za/public/sup/14419-a.pdf) and data were imported into REDCap (Research Electronic Data Capture).[7] Bowel preparation prior to colonoscopy was prescribed according to the endoscopist's preference and was not standardised for the OEU. Adequacy of bowel preparation at the time of colonoscopy was assessed by the endoscopist using a non-standardised score, graded as: (i) good; (ii) fair; (iii) poor; and (iv) bad. The variables collected enabled the calculation of caecal intubation rates (CIR) and PDR. We did not record any information on size, grade, histological type, morphology and anatomical location of polyps or detail regarding the technique of removal (biopsy v. endoscopic mucosal resection). To calculate ADR, histological reports were accessed to confirm whether polyps were adenomatous. In the analysis, we further stratified PDR and ADR by sex, age, population group, withdrawal time and bowel preparation. We analysed data overall and per endoscopist if a minimum of 50 colonoscopies were performed during the review period.
Definitions
CIR: number of colonoscopies where the caecum or terminal ileum was reached, divided by the number of colonoscopies where the intended endpoint was the caecum or terminal ileum; PDR: number of screening colonoscopies in which polyps were detected, divided by the total number of screening colonoscopies; and ADR: number of screening colonoscopies in which an adenoma was detected, divided by the total number of screening colonoscopies.
Benchmarks
CIR: >90% overall, and >95% for screening coloscopies; adequate outpatient bowel preparation rate: >85%; average withdrawal time for negative-result screening colonoscopies: >6 minutes; ADR: >25% overall (>30% for men and >20% for women);[2] PDR: >40% for men and >30% for women (there is no definitive benchmark for PDR from the ASGE/ACG Task Force or other governing bodies, such as the British Society of Gastroenterology (BSG) or the Joint Advisory
Group on gastrointestinal endoscopy (JAG). However, Williams et al.[8]concluded that a PDR of >40% for men and >30% for women was the minimum requirement to yield an ADR of >25% and >15% for men and women, respectively.[7,9]
Data analysis
CIR, PDR and ADR estimates were compared between patient groups by the x2 test; Fisher's exact test was used for 2 x 2 tables. Data analysis was done using SAS version 9.4 for Windows (Microsoft, USA). A p-value <0.05 was used.
Ethical approval
The study was approved by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand (ref. no. M171142).
Results
Demographic characteristics of patients undergoing colonoscopy
We recruited 1 643 patients from 1 March 2018 to 28 February 2019; their demographic characteristics are summarised in Table 1. The mean age of patients was 55.7 (standard deviation (SD) 14.4; range 18 -91) years, ~60% were female and the majority (75.5%) were white. Although this is an outpatient unit, 9.1% were hospital inpatients referred for colonoscopy to the OEU.

Indications for colonoscopy
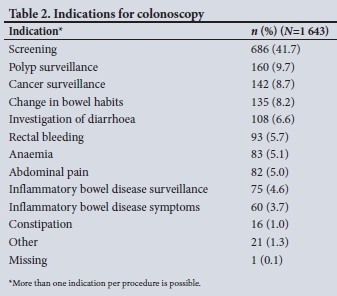
The most common indications for colonoscopy were screening (41.7%), polyp surveillance (9.7%) and cancer surveillance (8.7%) (Table 2).
Quality indicators for colonoscopy
Overall and screening CIR, adequacy of bowel preparation and withdrawal time on screening colonoscopy are shown in Table 3.
For screening colonoscopies, PDR and ADR stratified by sex, population group and withdrawal time are described in Table 4. Benchmarks for each quality indicator were included, where available.[2] We demonstrated a 23.7% increase in PDR and 14.1% increase in ADR between scopes that had mean withdrawal times of >6 minutes and <6 minutes. Of the 686 screening colonoscopies performed, 176 polyps/specimens were sent for histological analysis, and in these, 1 malignancy was diagnosed (grade 1 neuroendocrine tumour).
We were able to compare the performance of 10 of the 14 endoscopists who participated in the study - each had completed >50 colonoscopies during the study period. All of these endoscopists achieved an overall CIR above the benchmark of 90%; half (5/10) met the benchmark of >95% for screening colonoscopies; 2 achieved the >85% benchmark for adequate outpatient bowel preparation; 3 had a median withdrawal time of >6 minutes for negative-result screening colonoscopies; and only 1 met the benchmark of >40% and >30% for men and women, respectively, for PDR, and >25% for ADR.
Discussion
This preliminary clinical audit was our first attempt to evaluate the practice of colonoscopy in the OEU, with results proving promising for the future performance of the unit. It was expected that the majority of the scopes would be for screening (41.7%), surveillance for polyps (9.7%) and cancer (8.7%), as some of the endoscopists perform screening colonoscopies as part of the standard requirements for liver transplantation (WDGMC runs the largest liver transplant programme and has one of the largest oncology units in SA). Additionally, international guidelines recommend screening colonoscopy for those >50 years of age.[2] Therefore, it is reasonable that, when compared with an audit from Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) and international audits from Canada, the UK and Greece, the proportion of screening colonoscopies done at WDGMC was almost double (WDGMC: 41.7% v. 9.7% - 28.7%).[1,5,10-12]
When assessing the key quality indicators of screening colonoscopies, results showed that our PDR (27.2%) and ADR (15.6%) were low compared with international benchmarks and published data from high-income countries. These studies reported PDRs between 32.1% and 59.7%, and ADRs between 27.1% and 54.0%.[1,10,13-15] The obvious explanation might be that our patient profile is different, requiring locally derived, population-appropriate benchmarks for South Africans and, preferably, the sub-Saharan African (sSA) region. While a small proportion of patients in this clinical audit were black Africans, it is noteworthy that PDRs and ADRs for this group were similar to those of other population groups, despite the historical belief that black Africans 'do not get polyps'.[16] When compared with neighbouring countries, a study from Harare, Zimbabwe reported polyps in 5% of black Zimbabweans compared with 8% of white Zimbabweans.[17] Studies from Zambia and Nigeria reported overall PDRs of 10% and 7.4%, respectively.[18,19] Unfortunately, none of the studies from our region reported ADRs, as very few of the colonoscopies performed were true screening scopes. For the same reason, these PDRs must be interpreted with caution. The audit from CMJAH reported a PDR of 25.2%, which is similar to our PDR of 27.2%.[5] Overall, the higher PDR reported for different sites in Johannesburg - the largest and wealthiest urban metropole in the country - might reflect relative affluence, and evidence of an epidemiological sociodemographic transition from rural to urban living for its residents, with associated dietary and lifestyle changes. SA might be at a more advanced stage of this transition than other sSA countries. Interestingly, it is well documented that African Americans, who have similar genetic origins to black Africans, have a higher prevalence of polyps than their white counterparts, suggesting a gene-environment interaction that might alter clinical phenotypes.[20]
Alternatively, we could also question the quality of our clinical practice. Average withdrawal times >6 minutes were significantly associated with higher PDR/ADR and our withdrawal time on negative-result screening colonoscopies was just under the recommended target of >6 minutes. Although some endoscopists challenge the association between withdrawal times and PDR/ADR (as increasing withdrawal time alone will not improve an ADR/PDR that is already adequate), data suggest that longer withdrawal times are strongly associated with an increase in PDR and ADR, particularly when endoscopists do not achieve accepted benchmarks.[1,13,19,21]
Another factor that could have affected our PDR/ADR was the adequacy of our outpatient bowel preparation, which was below the ASGE/ACG Task Force benchmark of >85%. Interestingly, our results showed that inadequate bowel preparation yielded similar PDRs and ADRs to adequate bowel preparation, which could indicate that we are 'over-calling' poor bowel preparation as opposed to it actually being inadequate.[1,2] This could be owing to our clinicians not prescribing a standardised bowel preparation product or not using the same bowel preparation assessment tool when reporting the adequacy of this metric.
Despite not meeting the benchmark for bowel preparation, CIR was above the ASGE/ACG Task Force recommendation of >90% for all colonoscopies and >95% for screening colonoscopies. International audits from Canada, the UK and New Zealand report CIRs on par with ours.[1,11,13] It must be noted that in our calculation of CIR, we accounted for the starting intention of the colonoscopy, whereas other studies could have used the total number of screening colonoscopies, regardless of the intention. Other audits from SA report variable CIRs, both above and below the ASGE/ACG benchmark.[5,22]
As part of the continuation of this audit in years to come, we shared our overall results with all healthcare workers in the OEU, which stimulated productive discussion regarding areas for improvement and additional variables for inclusion. For example, we will implement the Harefield cleansing scale for assessing bowel preparation.[23] For results per endoscopist, the clinicians requested open sharing of their data in an anonymised format. Each endoscopist also received a confidential report of their performance metrics. The published literature suggests that confidential feedback to each individual on their metrics, without unit-wide intervention, makes a difference to PDR and withdrawal time.[24-26] On this basis, the endoscopists at WDGMC agreed to use results from this audit as a baseline for comparison in the next annual audit. The annual report is accessible in the public domain.
Study limitations
This audit is limited by the patient pool, as it reflects an urban, single-centre experience of health-insured individuals, and our findings may not be generalisable. The sample may be biased because the OEU screens all patients receiving liver transplants at WDGMC. Therefore, the screening population may not be average-risk asymptomatic individuals. There are no national guidelines or benchmarks for South Africans, or for different population groups within SA, which make interpreting low PDRs and ADRs difficult.
Conclusions
The audit of the WDGMC OEU revealed that 77.6% of outpatients had adequate bowel preparation, the CIR was 97.0% overall, and 96.3% for screening colonoscopies. The median withdrawal time for negative-result screening colonoscopies was 5.7 minutes. For screening colonoscopies, the PDR and ADR were 27.6% and 15.6%, respectively, which are lower than benchmarks from HIC regions with a 3-fold higher incidence of CRC, yet relatively higher than rates reported from other sSA countries. While short withdrawal times might affect the PDR and ADR, the validity of using externally derived high-income population benchmarks in SA deserves scrutiny. Given there are no standardised national benchmarks and guidelines on colonoscopy in SA, we hope these data will contribute to developing local guidelines. Annual auditing of clinical practice, and these data thus being available in the public domain, will be standard of care in our OEU, therefore making this year's audit a baseline for longitudinal observation and assessing the impact of interventions, as highlighted in our recommendations.
Recommendations
The clinicians have agreed to standardise education of patients regarding bowel preparation (with input from clinicians who achieve good bowel preparation rates) on the choice of bowel preparation and the use of a validated bowel preparation score.[27] Although adequate bowel preparation may seem straightforward, increasing patient adherence may incur a cost, such as employing a nurse educator who would be responsible for proper education of patients.[28] Furthermore, increasing withdrawal time should be strongly considered as a straightforward and relatively low-cost intervention. Due to the resource-intense nature of recording histological results for calculation of the ADR, the creation of an average adenoma-to-polyp detection rate quotient for the OEU could be cost-effective and useful in determining ADR from PDR, without needing these results. This will be feasible in time, as data from each annual audit can be used as a local reference.
Declaration. None.
Acknowledgements. We would like to thank Sister Gladys Kgabage and her team at the WDGMC OEU for their contribution to the study.
Author contributions. CB: wrote the first draft of the paper, contributed to the revision, and edited the final draft of the paper; PB, DB, BB, JD, NH, CJ, AM, JO, JR, MS, DS, DL: contributed to data collection and edited the final draft of the paper; GK: supervised data collection by the OEU staff and contributed to data collection; PG: contributed to planning and executing the statistics, and edited revisions and the final draft of the paper; HE: contributed to editing the drafts, the revisions and the final draft of the paper; JF: contributed to conceptualising the study, edited the first draft, subsequent revisions and final version of the paper; KK: conceptualised the study, contributed to the revision, contributed to data collection and edited the final draft of the paper.
Funding. Funding was provided by the WDGMC.
Conflicts of interest. None.
References
1. Gavin DR, Valori RM, Anderson JT, Donnelly MT, Williams JG, Swarbrick ET. The national colonoscopy audit: A nationwide assessment of the quality and safety of colonoscopy in the UK. Gut 2013;62(2):242-249. https://doi.org/10.1136/gutjnl-2011-301848 [ Links ]
2. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015;81(1):31-53. https://doi.org/10.1016/j.gie.2014.07.058 [ Links ]
3. Anderson JC, Butterly LF. Colonoscopy: Quality indicators. Clin Transl Gastroenterol 2015;6(2):e77. https://doi.org/10.1038/ctg.2015.5 [ Links ]
4. Leslie A, Carey FA, Pratt NR, Steele RJC. The colorectal adenoma-carcinoma sequence. Br J Surg 2002;89(7):845-860. https://doi.org/10.1046/j.1365-2168.2002.02120.x [ Links ]
5. Mahomed AD, Cremona E, Fourie C, et al. A clinical audit of colonoscopy in a gastroenterology unit at a tertiary teaching hospital in South Africa. S Afr Gastroenterol Rev 2012;10(3). [ Links ]
6. Cabebe EC, Espat J. What are the US multi-society task force on colorectal cancer guidelines for colorectal cancer screening tests? Medscape 2018. https://www.medscape.com/answers/2500006-108967/what-are-the-us-multi-society-task-force-on-colorectal-cancer-guidelines-for-colorectal-cancer-screening-tests (accessed 9 October 2020). [ Links ]
7. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377-381. https://doi.org/10.1016/j.jbl2008.08.010 [ Links ]
8. Williams JE, Holub JL, Faigel DO. Polypectomy rate is a valid quality measure for colonoscopy: Results from a national endoscopy database. Gastrointest Endosc 2012;75(3):576-582. https://doi.org/10.1016/j.gie.2011.12.012 [ Links ]
9. Williams JE, Le TD, Faigel DO. Polypectomy rate as a quality measure for colonoscopy. Gastrointest Endosc 2011;73(3):498-506. https://doi.org/10.1016/j.gie.2010.08.008 [ Links ]
10. Karamaroudis S, Stamou A, Vorri SC, et al. Monitoring of colonoscopy quality indicators in an academic endoscopy facility reveals adherence to international recommendations. Ann Transl Med 2018;6(13). https://doi.org/10.21037/atm.2018.03.34 [ Links ]
11. Armstrong D, Hollingworth R, Macintosh D, et al. Point-of-care, peer-comparator colonoscopy practice audit: The Canadian Association of Gastroenterology quality program - endoscopy. Can J Gastroenterol 2011;25(1):13-20. https://doi.org/10.1155/2011/320904 [ Links ]
12. De Jonge V, Sint Nicolaas J, Lalor EA, et al. A prospective audit of patient experiences in colonoscopy using the global rating scale: A cohort of 1 187 patients. Can J Gastroenterol 2010;24(10):607-613. https://doi.org/10.1155/2010/724924 [ Links ]
13. Fraser AG, Gamble GD, Rose TR, Dunn JP. Colonoscopy audit over 10 years - what can be learnt? N Z Med J 2013;126(1382):25-35. [ Links ]
14. Lee TJW, Rutter MD, Blanks RG, et al. Colonoscopy quality measures: Experience from the NHS bowel cancer screening programme. Gut 2012;61(7):1050-1057. https://doi.org/10.1136/gutjnl-2011-300651 [ Links ]
15. Rees CJ, Thomas Gibson S, Rutter MD, et al UK key performance indicators and quality assurance standards for colonoscopy. Gut 2016;65(12):1923-1929. https://doi.org/10.1136/gutjnl-2016-312044 [ Links ]
16. Segal I, Cooke SA, Hamilton DG, Ou Tim L. Polyps and colorectal cancer in South African Blacks. Gut 1981;22(8):653-657. http://doi.org/10.1136/gut.22.8.653 [ Links ]
17. Katsidzira L, Gangaidzo IT, Mapingure MP, Matenga JA. Retrospective study of colorectal cancer in Zimbabwe: Colonoscopic and clinical correlates. World J Gastroenterol 2015;21(8):2374-2380. https://doi.org/10.3748/wjg.v21.i8.2374 [ Links ]
18. Osinowo A, Lawal O, Lesi O, Olajide T, Adesanya A. Audit of colonoscopy practice in Lagos University Teaching Hospital. J Clin Sci 2016;13(1):29. https://doi.org/10.4103/1595-9587.175487 [ Links ]
19. Kayamba V, Nicholls K, Morgan C, Kelly P. A seven-year retrospective review of colonoscopy records from a single centre in Zambia. Malawi Med J 2018;30(1):17-21. https://doi.org/10.4314/mmj.v30i1.4 [ Links ]
20. Lieberman D, Holub J, Moravec M, Eisen G, Peters D, Morris C. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA 2008;300(12):1417-1422. https://doi.org/10.1001/jama.300.12.1417 [ Links ]
21. Calderwood AH, Jacobson BC. Colonoscopy quality: Metrics and implementation. Gastroenterol Clin North Am 2013;42(3):599-618. https://doi.org/10.1016/j.gtc.2013.05.005 [ Links ]
22. Benamro A, Bruce JL, Clarke DL. An electronic colonoscopy record system enables detailed quality assessment and benchmarking of an endoscopic service. S Afr Med J 2015;105(12):1061. https://doi.org/10.7196/SAMJ.2015.v105i12.10115 [ Links ]
23. Halphen M, Heresbach D, Gruss H-J, Belsey J. Validation of the Harefield cleansing scale: A tool for the evaluation of bowel cleansing quality in both research and clinical practice. Gastrointest Endosc 2013;78(1):121-131. https://doi.org/10.1016/j.gie.2013.02.009 [ Links ]
24. Lin OS, Kozarek RA, Arai A, et al The effect of periodic monitoring and feedback on screening colonoscopy withdrawal times, polyp detection rates, and patient satisfaction scores. Gastrointest Endosc 2010;71(7):1253-1259. https://doi.org/10.1016/j.gie.2010.01.017 [ Links ]
25. Gurudu SR, Boroff ES, Crowell MD, et al. Impact of feedback on adenoma detection rates: Outcomes of quality improvement program. J Gastroenterol Hepatol 2018;33(3):645-649. https://doi.org/10.1111/jgh.13984 [ Links ]
26. Nielsen AB, Nielsen OH, Hendel J. Impact of feedback and monitoring on colonoscopy withdrawal times and polyp detection rates. BMJ Open Gastroenterol 2017;4(1):e000142. https://doi.org/10.1136/bmjgast-2017-000142 [ Links ]
27. Kastenberg D, Bertiger G, Brogadir S. Bowel preparation quality scales for colonoscopy. World J Gastroenterol 2018;24(26):2833-2843. https://doi.org/10.3748/wjg.v24.i26.2833 [ Links ]
28. Rosenfeld G, Krygier D, Enns RA, Singham J, Wiesinger H, Bressler B. The impact of patient education on the quality of inpatient bowel preparation for colonoscopy. Can J Gastroenterol 2010;24(9):543-546. https://doi.org/10.1155/2010/718628 [ Links ]
 Correspondence:
Correspondence:
C Bouter
carolynbouter@outlook.com
Accepted 3 June 2020














